Question: please help me out, I do not understand Based only on their intermolecular forces, which of the following pairs of molecules would be most soluble
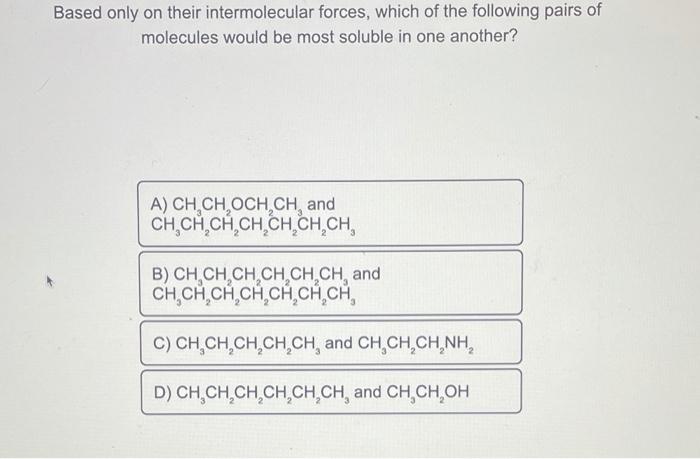
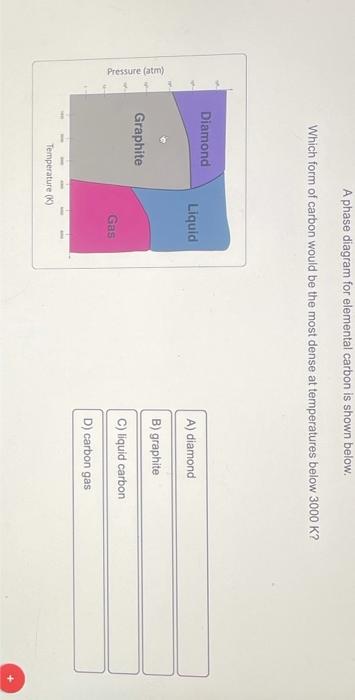
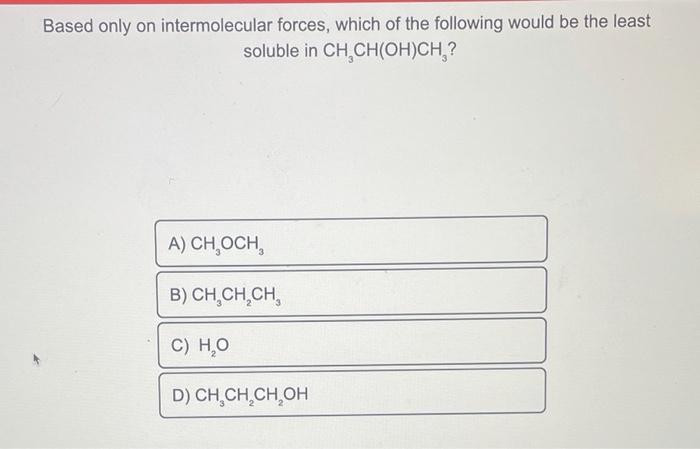
Based only on their intermolecular forces, which of the following pairs of molecules would be most soluble in one another? A phase diagram for elemental carbon is shown below. Which form of carbon would be the most dense at temperatures below 3000K ? Based only on intermolecular forces, which of the following would be the least solubleinCH3CH(OH)CH3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


