Question: please help me plot this graph and answer these questions Freezing Points-Graphing of Data Data Table Pure Acetic Acid Pure Acetic Acid Impure Acetic Acid
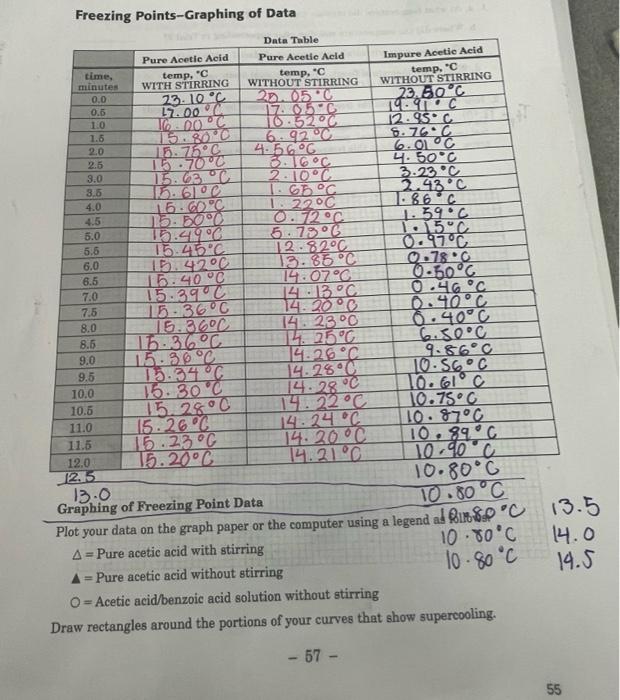
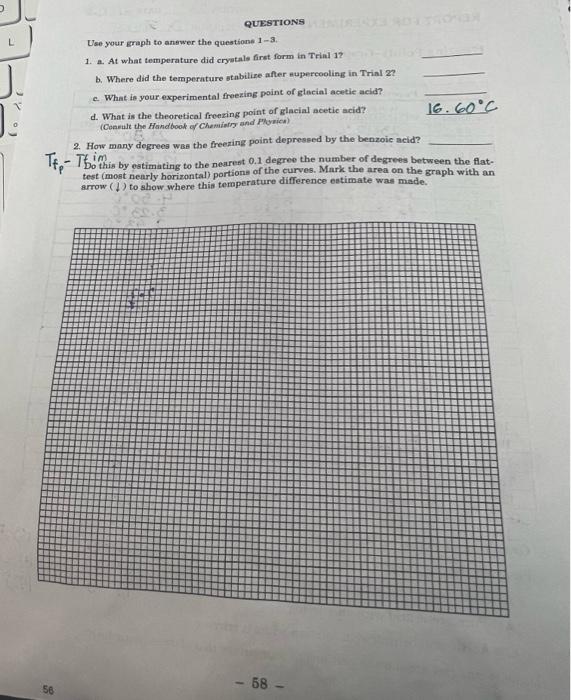

Freezing Points-Graphing of Data Data Table Pure Acetic Acid Pure Acetic Acid Impure Acetic Acid time, temp. C temp. C temp. C minutes WITH STIRRING WITHOUT STIRRING WITHOUT STIRRING 0.0 23.10C 22.050 23.60C 0.8 17.00 17.05C 14.DOC 10 16.00 C 10:5200 12.95C 1.5 15.800 6.9200 6.766 2.0 15.75C 4.56C 6.01 C 2.5 15.70C 3.16C 4.500 3.0 15.63 C 2.10C 3.23 C 3.6 15.610C 1.66C. 2.43C 4.0 15. GDPC 1. 2200 1586C 4.5 25.500 0.72C 1.59.c 5.0 15.4.9C 5.78 C 1. 15C 5.6 15.45C 12.82C 0.97C 6.0 15.4200 13.85C 0.780 6.5 15.40 C 14.07C 0.60C 7.0 15.390C 14:13 C 0.46C 7.5 15.36C 14. 20C 0.40C 8.0 16.36C 14. 23C 0.40C 8.5 16.36C L. 25C 6.50C 9.0 15.36C 14.26 C 9.86C 9.5 15-34C 14.25C 10.56C 10.0 15. 30C 14.28 10. El C 10.5 15.28C 145.22 C 10.75C 11.0 15.26 C 14.24 C 10.87C 11.5 15.23 C 14. 20C 89C 12.0 15.20C 14.21C 10.90 c 12.5 10.80C 13.0 Graphing of Freezing Point Data 10.80C Plot your data on the graph paper or the computer using a legend al Rub&P 'C 13.5 A = Pure acetic acid with stirring 10.80C 14.0 A = Pure acetic acid without stirring 10.80C 14.5 O=Acetic acid/benzoic acid solution without stirring Draw rectangles around the portions of your curves that show supercooling. C 10. - 57 - 55 QUESTIONS Use your graph to answer the questions 1-3 1. a. At what temperature did crystals first form in Trial 17 b. Where did the temperature stabilire after supercooling in Trial 22 c. What is your experimental freezing point of glacial acetie acid? d. What is the theoretical freezing point of glacial acetic acid? 16.60C (Consult the Handbook of Chemistry and Planica) 2. How many degrees was the freezing point depressed by the benzoic acid? Do this by estimating to the nearest 0.1 degree the number of degrees between the flat- test (most nearly horizontal) portions of the curves. Mark the area on the graph with an arrow () to show where this temperature difference estimate was made. TE- TEAM 58 - 56 REPORT FOR EXPERIMENT (continued) NAME 3. . What is the effect of stirring on the freezing point of pure scetic acid b. What is the effect of stirring on supereeling! 4. . What do the melting point and freezing point of a substance have in commen? b. What is the difference between the melting and freezing of a substance? 5. When the solid and liquid phases are in equilibrium, which phase, solid or liquid contains the greater amount of energy? Explain the rationale for your answer. - 59
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


