Question: Please help me solve and explain the question given. (Don't copy/spam the answer) 1. If the molar volume of a binary mixture is given by
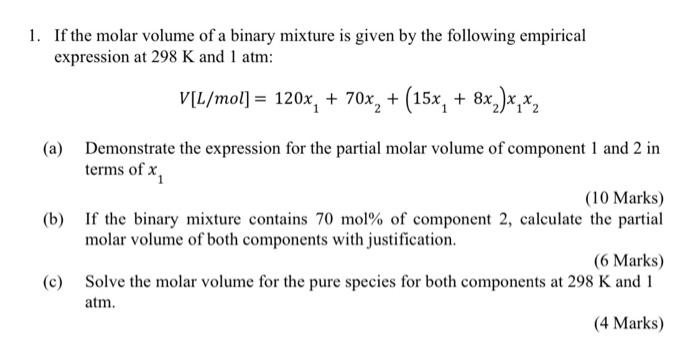
1. If the molar volume of a binary mixture is given by the following empirical expression at 298 K and 1 atm: V[L/mol] = 120x, + 70x2 + (15x, + 8x_)x,x2 (a) Demonstrate the expression for the partial molar volume of component 1 and 2 in terms of x (10 Marks) (b) If the binary mixture contains 70 mol% of component 2, calculate the partial molar volume of both components with justification. (6 Marks) (c) Solve the molar volume for the pure species for both components at 298 K and 1 atm. (4 Marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


