Question: please help me with my exam!! 6. Ammonia breaks down into nitrogen and hydrogen according to the following chemical equation: 2NH3(g)N2(g)+3H2(g) When 12.0 moles of
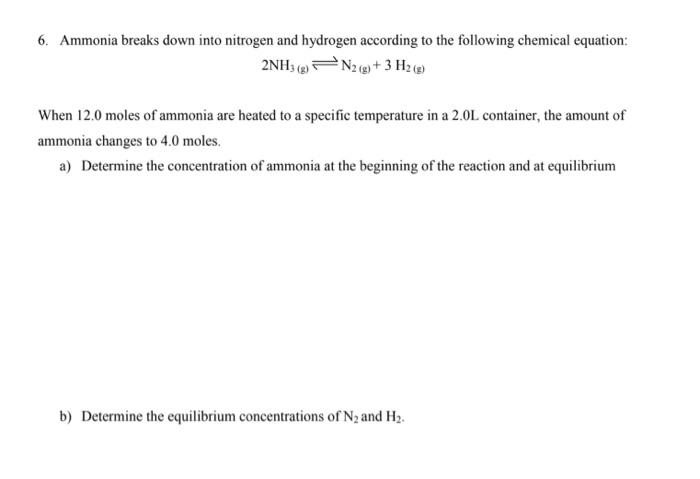
6. Ammonia breaks down into nitrogen and hydrogen according to the following chemical equation: 2NH3(g)N2(g)+3H2(g) When 12.0 moles of ammonia are heated to a specific temperature in a 2.0L container, the amount of ammonia changes to 4.0 moles. a) Determine the concentration of ammonia at the beginning of the reaction and at equilibrium b) Determine the equilibrium concentrations of N2 and H2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


