Question: please help me with part B 2. Interpret: The salt produced by the reaction of acetic acid and sodium hydroxide, CH3COONa, is a weak base.
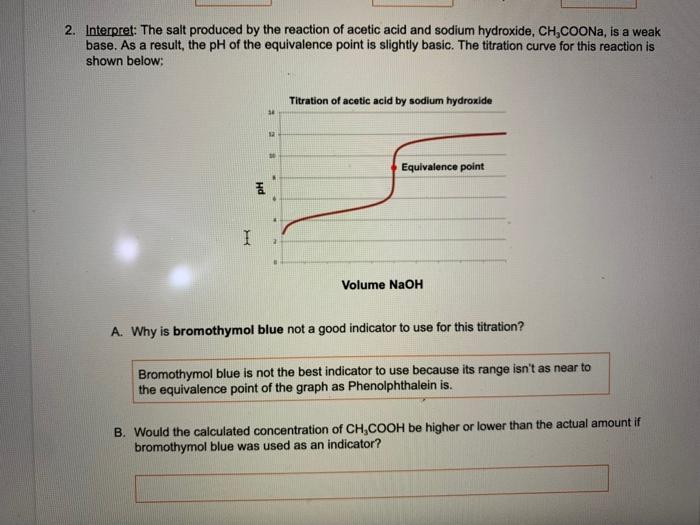
2. Interpret: The salt produced by the reaction of acetic acid and sodium hydroxide, CH3COONa, is a weak base. As a result, the pH of the equivalence point is slightly basic. The titration curve for this reaction is shown below: A. Why is bromothymol blue not a good indicator to use for this titration? Bromothymol blue is not the best indicator to use because its range isn't as near to the equivalence point of the graph as Phenolphthalein is. B. Would the caiculated concentration of CH3COOH be higher or lower than the actual amount if bromothymol blue was used as an indicator
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


