Question: please help Methanol (CH3OH) is produced commercially by the catalyzed reaction of carbon monoxide and hydrogen: Calculate Kc at this temperature. CO(g)+2H2(g)CH3OH(g) An equilibrium mixture
please help 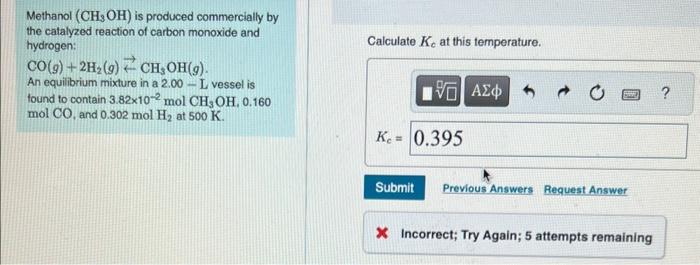

Methanol (CH3OH) is produced commercially by the catalyzed reaction of carbon monoxide and hydrogen: Calculate Kc at this temperature. CO(g)+2H2(g)CH3OH(g) An equilibrium mixture in a 2.00 - L vessel is tound to contain 3.82102molCH3OH,0.160 molCO, and 0.302molH2 at 500K. X Incorrect; Try Again; 5 attempts remaining
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


