Question: please help need this to pass the class! will give a thumbs up just need answers no work needed :) How many moles of carbon
please help need this to pass the class! will give a thumbs up just need answers no work needed :) 



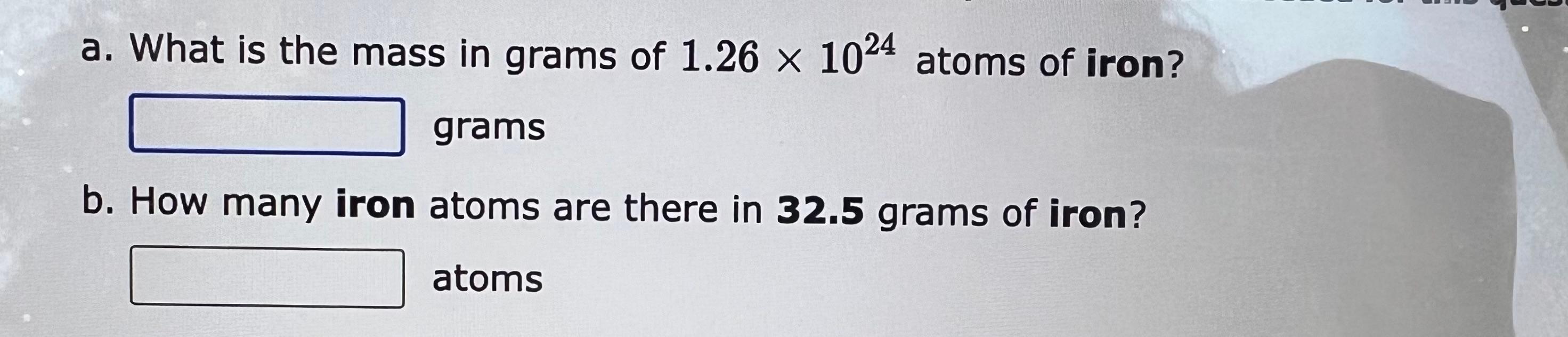


How many moles of carbon tetrachloride, CCl4, are present in 4.28 grams of this compound moles b. How many grams of carbon tetrachloride are present in 2.63 moles of this compound? grams a. How many grams of aluminum bromide, AlBr3, are present in 3.40 moles of this compound? grams b. How many moles of aluminum bromide are present in 2.67 grams of this compound? moles a. How many grams of sodium bromide, NaBr, are present in 4.48 moles of this compound? grams b. How many moles of sodium bromide are present in 1.87 grams of this compound? moles formula for carbon tetrachloride is CCl4. a. How many molecules are in 4.73 grams of carbon tetrachloride? molecules b. What is the mass in grams of 9.021022 molecules of carbon tetrachloride? grams a. What is the mass in grams of 1.261024 atoms of iron? grams b. How many iron atoms are there in 32.5 grams of iron? atoms a. What is the mass in grams of 1.761024 atoms of aluminum? grams b. How many aluminum atoms are there in 26.5 grams of aluminum? atoms sample of chlorine has a mass of 39.8 grams. a. How many Cl atoms are there in the sample? atoms b. How many Cl2 molecules are there in the sample? molecules
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


