Question: please help on this question a. 1. For each of the following molecules (in which the central atom is underlined), classify it according to the
please help on this question
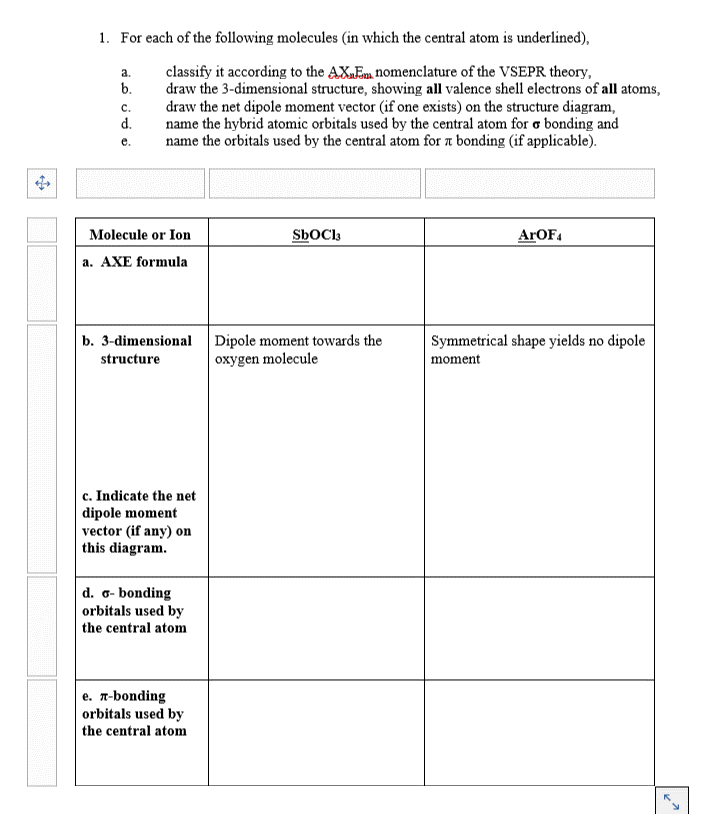
a. 1. For each of the following molecules (in which the central atom is underlined), classify it according to the AXnEm nomenclature of the VSEPR theory, b. draw the 3-dimensional structure, showing all valence shell electrons of all atoms, draw the net dipole moment vector (if one exists) on the structure diagram, d. name the hybrid atomic orbitals used by the central atom for a bonding and name the orbitals used by the central atom for a bonding (if applicable). c. e. SbOCI ArOF Molecule or Ion a. AXE formula b. 3-dimensional Dipole moment towards the structure oxygen molecule Symmetrical shape yields no dipole moment c. Indicate the net dipole moment vector (if any) on this diagram. d. 6-bonding orbitals used by the central atom e. a-bonding orbitals used by the central atom
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


