Question: please help question #2 2. One Step Further: Suppose 1.0109mol of radioiodine were released into a water reservoir after a nuclear accident. Approximately how many
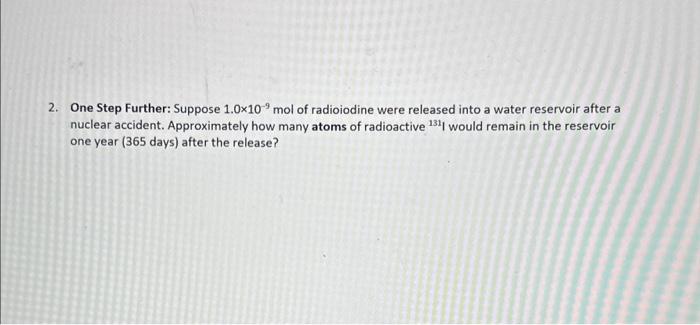

2. One Step Further: Suppose 1.0109mol of radioiodine were released into a water reservoir after a nuclear accident. Approximately how many atoms of radioactive 131 I would remain in the reservoir one year ( 365 days) after the release? 131 is a radioactive isotope (or radioisotope) of iodine with 53 protons and 78 neutrons in its nucleus. Its nuclear decay reaction proceeds by a first-order process that produces a 131 Xe nucleus, a particle (electron), an antineutrino (ve) and high-energy gamma rays: 53131I54131Xe+e+ve+t1/2=8.02days High exposure to 131 I radioactivity can cause damage to the thyroid, which is the main organ in the body that uses iodine. This can be a health danger after a nuclear disaster like the one at Fukushima, Japan in 2011 , because 131 is produced by the fission of uranium and plutonium and can contaminate air and water sources. For thyroid cancer patients, however, this effect can be a blessing in disguise-when they take a prescribed dose of 131, the thyroid cancer cells naturally absorb more of the radioactive element, which then kills the cancer with little damage to the rest of the body
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


