Question: An example of a simple, one-electron reaction on the surface of an electrode is the reduction of ferricyanide to ferrocyanide (Fe3+ in ferricyanide is reduced
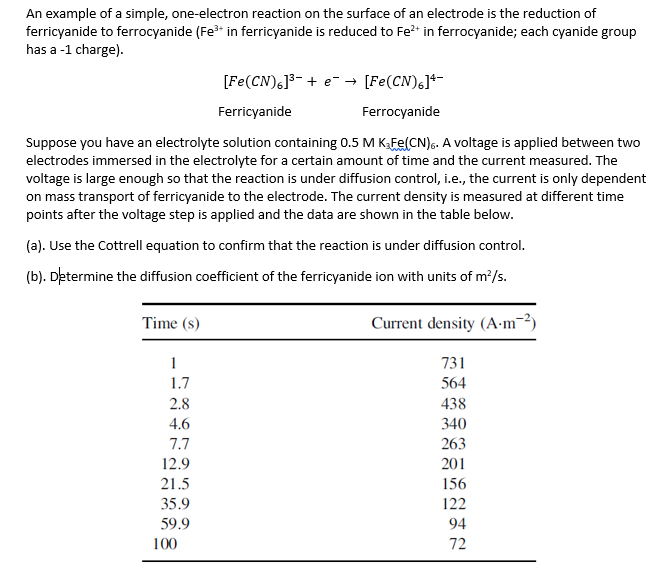
An example of a simple, one-electron reaction on the surface of an electrode is the reduction of ferricyanide to ferrocyanide (Fe3+ in ferricyanide is reduced to Fe2+ in ferrocyanide; each cyanide group has a -1 charge). [Fe(CN)6]3+eFerricyanide[Fe(CN)6]4Ferrocyanide Suppose you have an electrolyte solution containing 0.5MK3Fe(CN)6. A voltage is applied between two electrodes immersed in the electrolyte for a certain amount of time and the current measured. The voltage is large enough so that the reaction is under diffusion control, i.e., the current is only dependent on mass transport of ferricyanide to the electrode. The current density is measured at different time points after the voltage step is applied and the data are shown in the table below. (a). Use the Cottrell equation to confirm that the reaction is under diffusion control. (b). Determine the diffusion coefficient of the ferricyanide ion with units of m2/s
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


