Question: please help! Sodium hydroxide is a white, crystalline basic salt that readily dissociates in water to produce a strong base. NaOH(s)Na+(aq)+OH(aq) According to the CRC
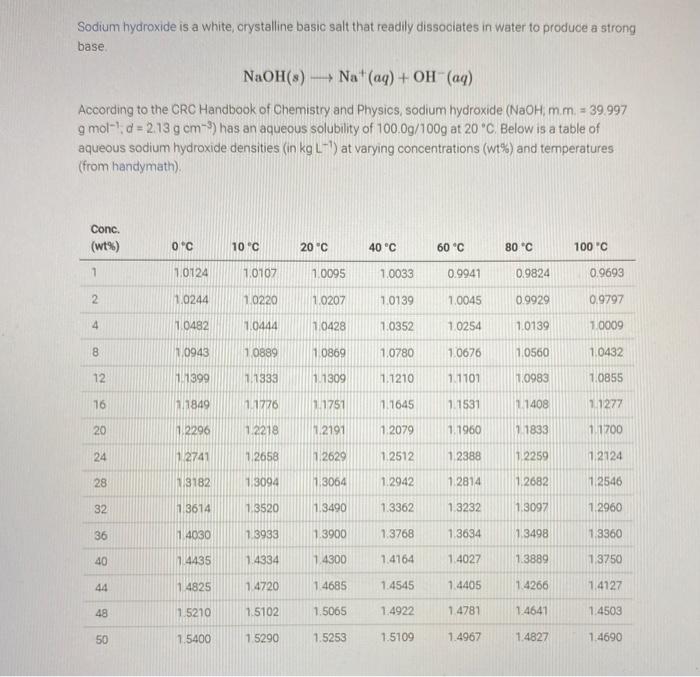
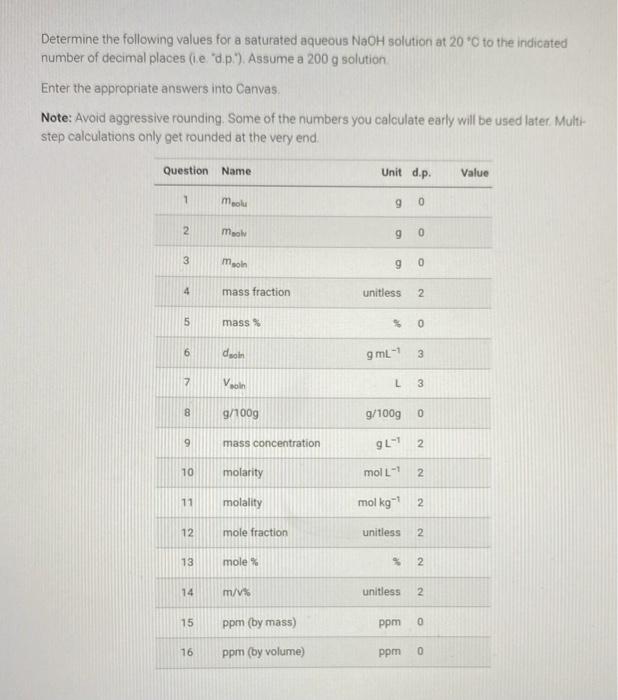
Sodium hydroxide is a white, crystalline basic salt that readily dissociates in water to produce a strong base. NaOH(s)Na+(aq)+OH(aq) According to the CRC Handbook of Chemistry and Physics, sodium hydroxide (NaOH,m.m. 39.997 gmol1,d=2.13gcm3 ) has an aqueous solubility of 100.0g/100g at 20C. Below is a table of aqueous sodium hydroxide densities (in kgL1 ) at varying concentrations (wt\%) and temperatures (from handymath). Determine the following values for a saturated aqueous NaOH solution at 20O to the indicated number of decimal places (i.e "d.p."). Assume a 200g solution. Enter the appropriate answers into Canvas. Note: Avoid aggressive rounding. Some of the numbers you calculate early will be used later. Multistep calculations only get rounded at the very end. Sodium hydroxide is a white, crystalline basic salt that readily dissociates in water to produce a strong base. NaOH(s)Na+(aq)+OH(aq) According to the CRC Handbook of Chemistry and Physics, sodium hydroxide (NaOH,m.m. 39.997 gmol1,d=2.13gcm3 ) has an aqueous solubility of 100.0g/100g at 20C. Below is a table of aqueous sodium hydroxide densities (in kgL1 ) at varying concentrations (wt\%) and temperatures (from handymath). Determine the following values for a saturated aqueous NaOH solution at 20O to the indicated number of decimal places (i.e "d.p."). Assume a 200g solution. Enter the appropriate answers into Canvas. Note: Avoid aggressive rounding. Some of the numbers you calculate early will be used later. Multistep calculations only get rounded at the very end
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


