Question: Please help solve this question, as my answer is incorrect or imputed incorrectly :) Zinc metal reacts with hydrochloric acid according to the following balanced
 Please help solve this question, as my answer is incorrect or imputed incorrectly :)
Please help solve this question, as my answer is incorrect or imputed incorrectly :)
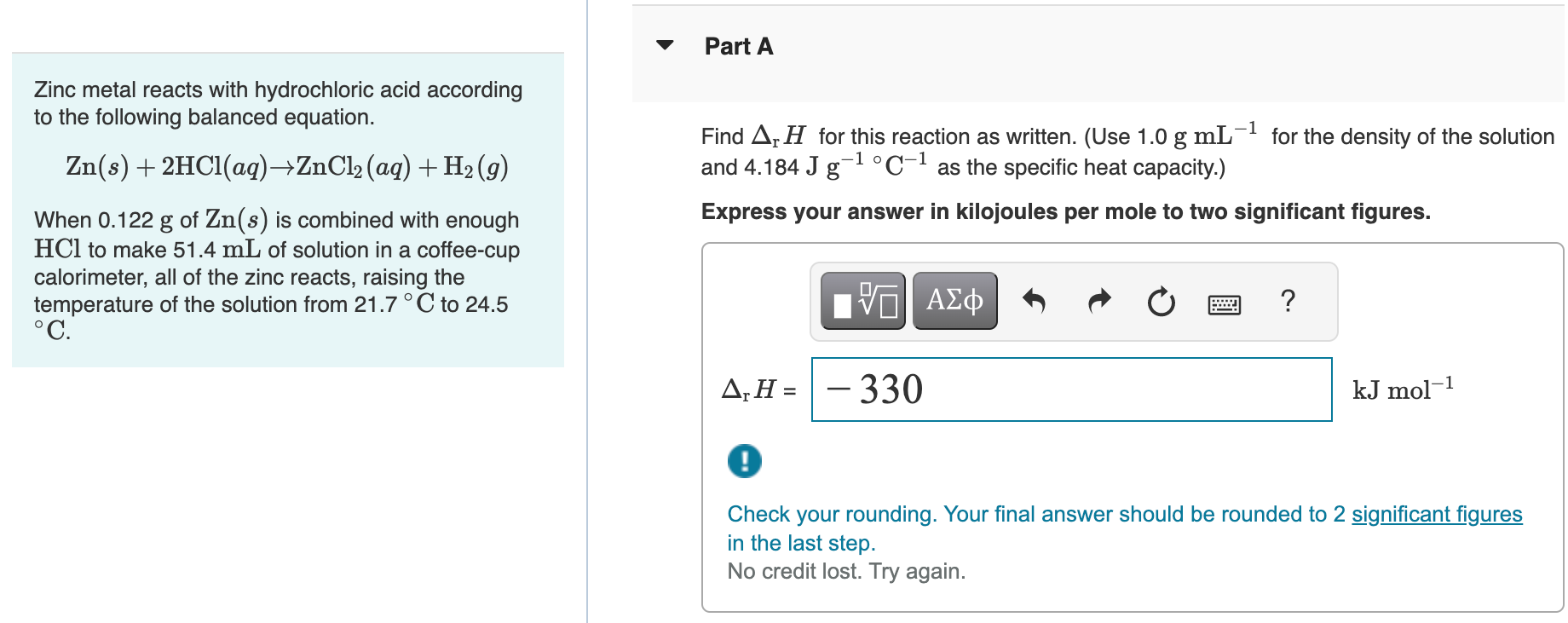
Zinc metal reacts with hydrochloric acid according to the following balanced equation. Zn(s)+2HCl(aq)ZnCl2(aq)+H2(g) and 4.184Jg1C1 as the specific heat capacity.) When 0.122g of Zn(s) is combined with enough Express your answer in kilojoules per mole to two significant figures. HCl to make 51.4mL of solution in a coffee-cup calorimeter, all of the zinc reacts, raising the temperature of the solution from 21.7C to 24.5 C. Check your rounding. Your final answer should be rounded to 2 significant figures in the last step. No credit lost. Try again
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


