Question: please help, the answer is 305torr and i keep getting 316 :( 60. Consider the reaction: CO(g)+H2O(g)CO2(g)+H2(g)Kp=0.0611at2000K A reaction mixture initially contains a CO partial
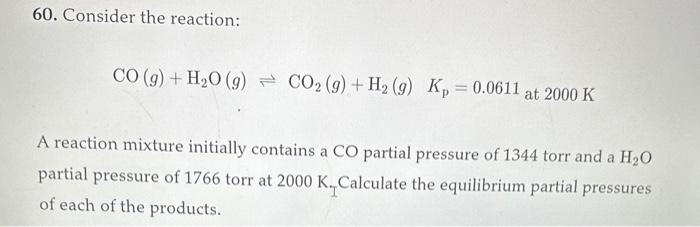
60. Consider the reaction: CO(g)+H2O(g)CO2(g)+H2(g)Kp=0.0611at2000K A reaction mixture initially contains a CO partial pressure of 1344 torr and a H2O partial pressure of 1766 torr at 2000K.II Calculate the equilibrium partial pressures of each of the products
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


