Question: please help!!!! This reaction was monitored as a function of time: ABA+B A plot of 1/[AB] versus time yields a straight line with slope 0.056molL1s1.
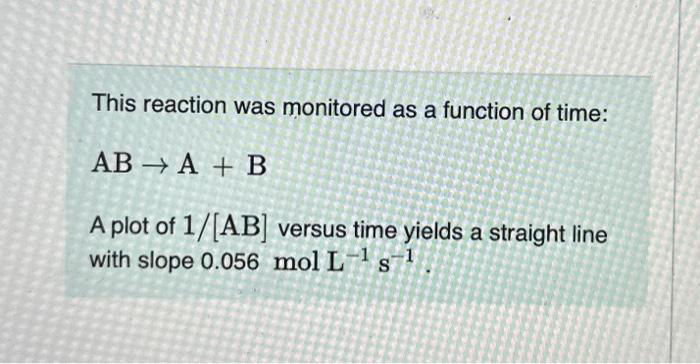
![ABA+B A plot of 1/[AB] versus time yields a straight line with](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f91b2a8dea6_45066f91b2a38875.jpg)
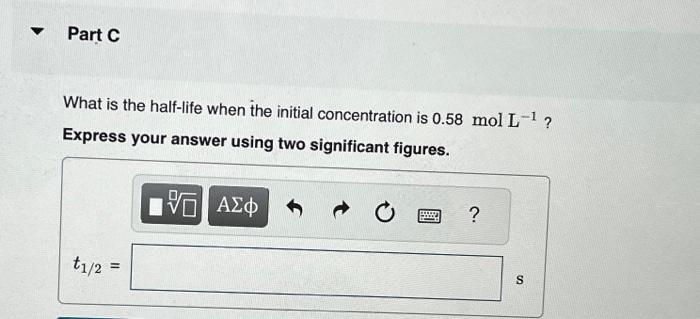

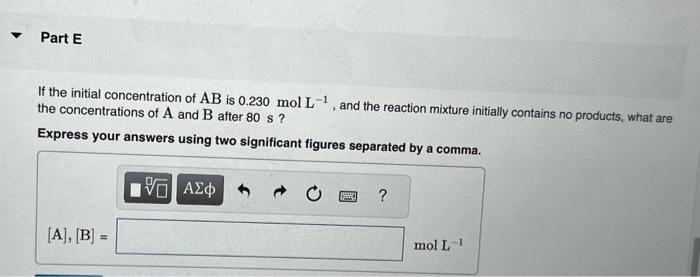
This reaction was monitored as a function of time: ABA+B A plot of 1/[AB] versus time yields a straight line with slope 0.056molL1s1. What is the value of the rate constant (k) for this reaction at this temperature? Express your answer using two significant figures. What is the half-life when the initial concentration is 0.58molL1 ? Express your answer using two significant figures. How long will it take for the concentration of AB to decrease from 0.58molL1 to 0.15molL1 ? Express your answer using two significant figures. If the initial concentration of AB is 0.230molL1, and the reaction mixture initially contains no products, what are the concentrations of A and B after 80s ? Express your answers using two significant figures separated by a comma
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


