Question: PLEASE HELP TO ANSWER ! thankyou 1. (a) A common ion selective electrode for Pb2+ is composed of a mixed Ag2S,PbS crystalline membrane. It is
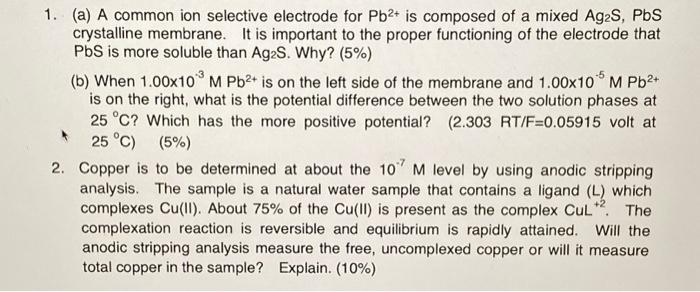
1. (a) A common ion selective electrode for Pb2+ is composed of a mixed Ag2S,PbS crystalline membrane. It is important to the proper functioning of the electrode that PbS is more soluble than Ag2S. Why? (5%) (b) When 1.00103MPb2+ is on the left side of the membrane and 1.00105MPb2+ is on the right, what is the potential difference between the two solution phases at 25C ? Which has the more positive potential? (2.303 RT/ F=0.05915 volt at 25C)(5%) 2. Copper is to be determined at about the 107M level by using anodic stripping analysis. The sample is a natural water sample that contains a ligand (L) which complexes Cu(II). About 75% of the Cu(II) is present as the complex CuL+2. The complexation reaction is reversible and equilibrium is rapidly attained. Will the anodic stripping analysis measure the free, uncomplexed copper or will it measure total copper in the sample? Explain. (10\%)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


