Question: please help to answer these pre lab questions from the pH of lots of stuff chem II lab. please lmk if you have any questions,
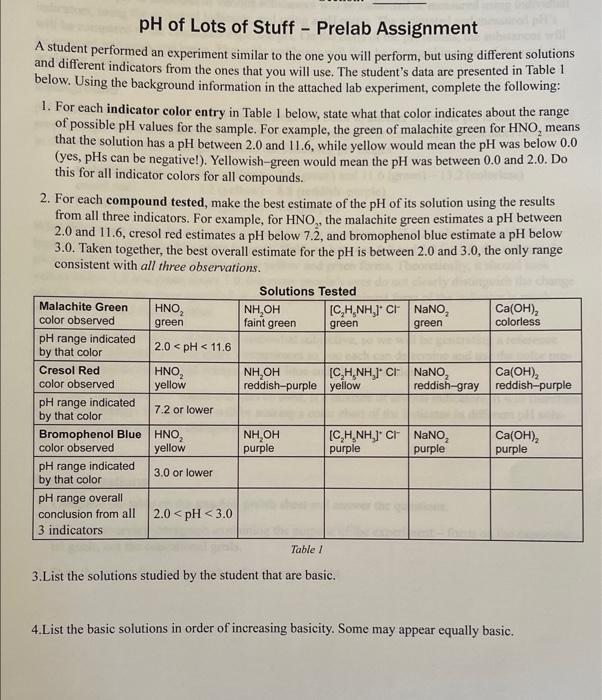
pH of Lots of Stuff - Prelab Assignment A student performed an experiment similar to the one you will perform, but using different solutions and different indicators from the ones that you will use. The student's data are presented in Table 1 below. Using the background information in the attached lab experiment, complete the following: 1. For each indicator color entry in Table I below, state what that color indicates about the range of possible pH values for the sample. For example, the green of malachite green for HNO, means that the solution has a pH between 2.0 and 11.6, while yellow would mean the pH was below 0.0 (yes, pHs can be negative!). Yellowish-green would mean the pH was between 0.0 and 2.0. Do this for all indicator colors for all compounds. 2. For each compound tested, make the best estimate of the pH of its solution using the results from all three indicators. For example, for HNO,, the malachite green estimates a pH between 2.0 and 11.6, cresol red estimates a pH below 7.2, and bromophenol blue estimate a pH below 3.0. Taken together, the best overall estimate for the pH is between 2.0 and 3.0, the only range consistent with all three observations. Solutions Tested Malachite Green HNO NH,OH [C,H.NH,]* CF NaNO, Ca(OH)2 color observed green colorless pH range indicated by that color 2.0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


