Question: Please help. Very lost on how to solve these. Thank you. For the reaction: H2(g)+Br2(g)2HBr(g) Kp=3.5104 at 1495K. What is the value of Kp for
Please help. Very lost on how to solve these. Thank you.
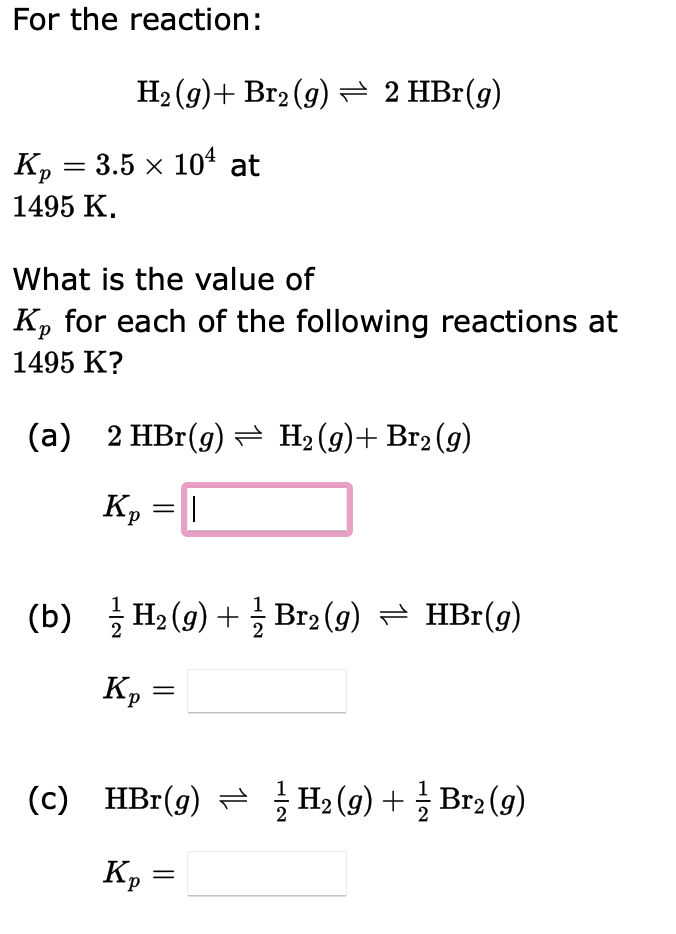
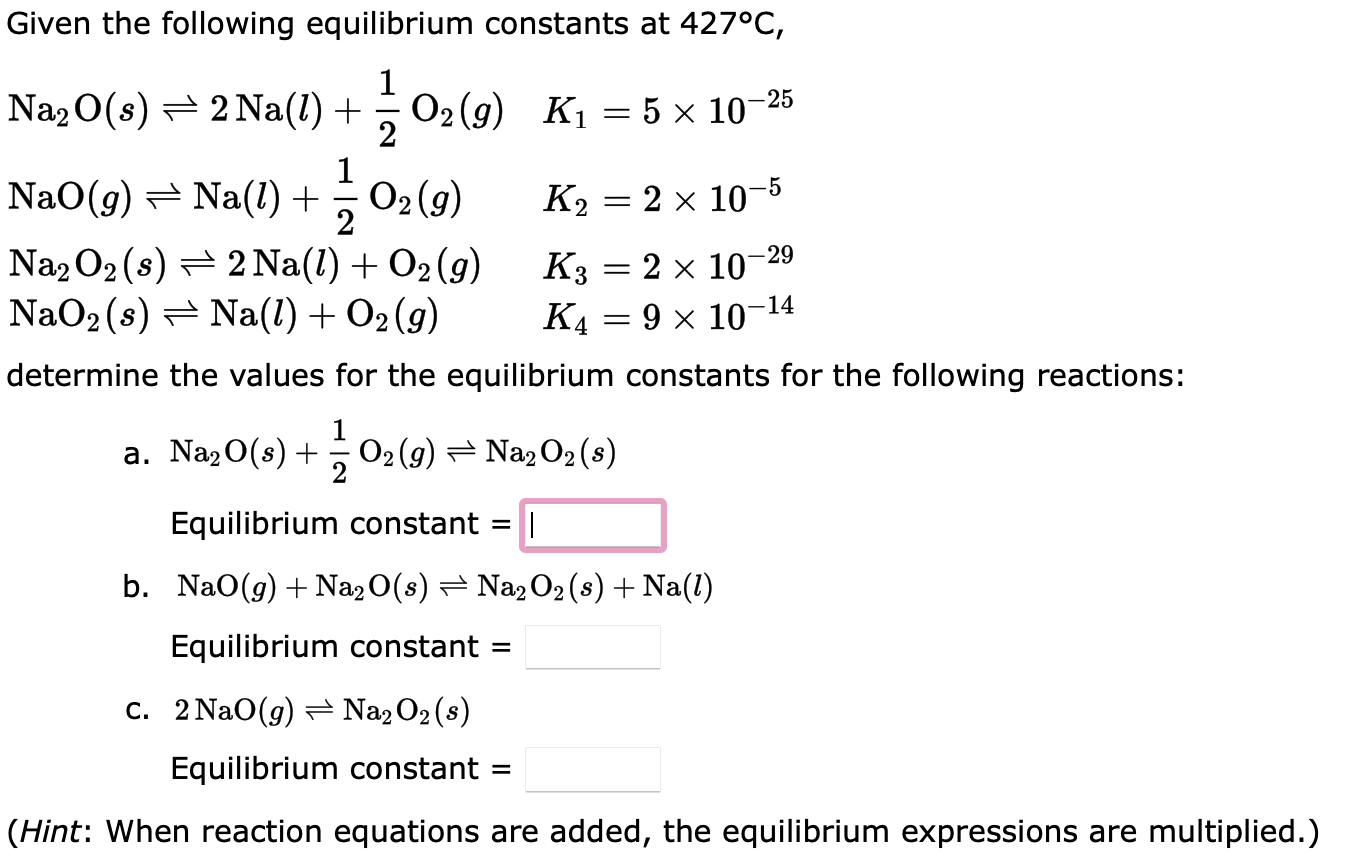
For the reaction: H2(g)+Br2(g)2HBr(g) Kp=3.5104 at 1495K. What is the value of Kp for each of the following reactions at 1495K ? (a) 2HBr(g)H2(g)+Br2(g) Kp= (b) 21H2(g)+21Br2(g)HBr(g) Kp= (c) HBr(g)21H2(g)+21Br2(g) Kp= Given the following equilibrium constants at 427C, Na2O(s)2Na(l)+21O2(g)NaO(g)Na(l)+21O2(g)Na2O2(s)2Na(l)+O2(g)NaO2(s)Na(l)+O2(g)K1=51025K2=2105K3=21029K4=91014 determine the values for the equilibrium constants for the following reactions: a. Na2O(s)+21O2(g)Na2O2(s) Equilibrium constant = b. NaO(g)+Na2O(s)Na2O2(s)+Na(l) Equilibrium constant = C. 2NaO(g)Na2O2(s) Equilibrium constant = (Hint: When reaction equations are added, the equilibrium expressions are multiplie
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


