Question: please help!! What is the value for K if the equilibrium pressures for hydrogen, oxygen, and water are 1,0.5, and 1atm, respectively? 2H2(g)+O2(g)2H2O(g) Hint. K=PH22PO2PH2O3
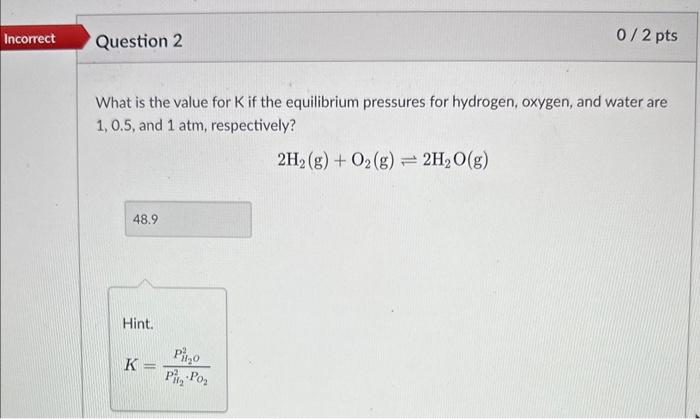
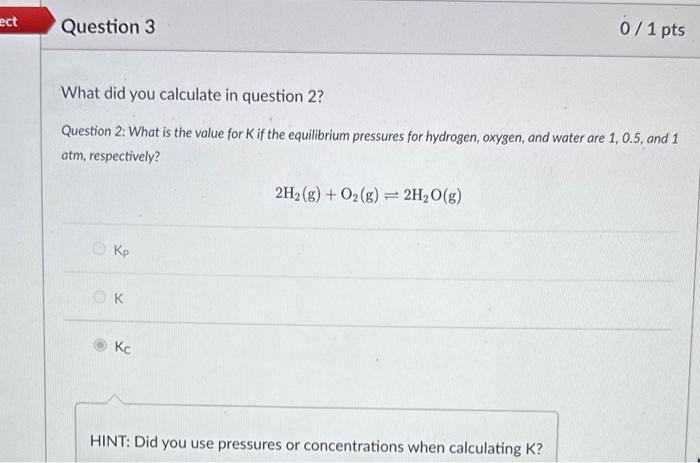
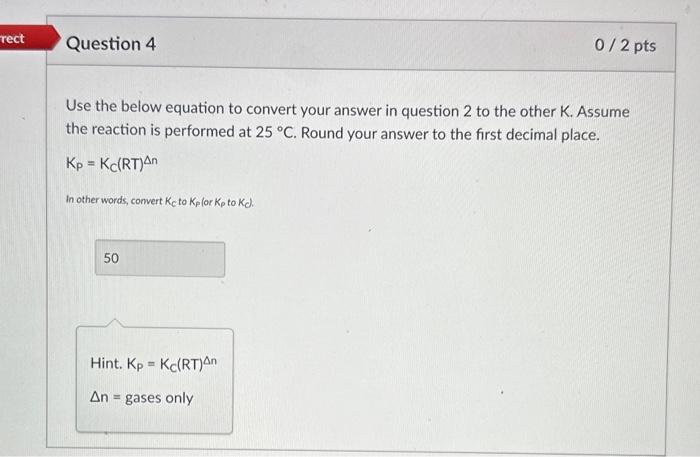
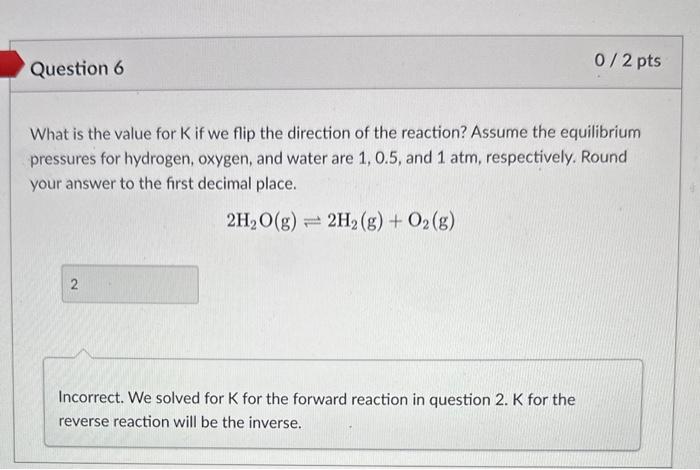
What is the value for K if the equilibrium pressures for hydrogen, oxygen, and water are 1,0.5, and 1atm, respectively? 2H2(g)+O2(g)2H2O(g) Hint. K=PH22PO2PH2O3 Question 2: What is the value for K if the equilibrium pressures for hydrogen, oxygen, and water are 1,0.5, and 1 atm, respectively? 2H2(g)+O2(g)2H2O(g) Kp K KC HINT: Did you use pressures or concentrations when calculating K? Use the below equation to convert your answer in question 2 to the other K. Assume the reaction is performed at 25C. Round your answer to the first decimal place. Kp=Kc(RT)n In other words, convert Kc to Kp lor Kp to Kd. Hint. Kp=KC(RT)n n= gases only What is the value for K if we flip the direction of the reaction? Assume the equilibrium pressures for hydrogen, oxygen, and water are 1,0.5, and 1atm, respectively. Round your answer to the first decimal place. 2H2O(g)2H2(g)+O2(g) Incorrect. We solved for K for the forward reaction in question 2 . K for the reverse reaction will be the inverse
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


