Question: Please help! Will Upvote! I'm not sure how to interpret this data!! Thank you Titration of 1.1340g of an unknown acid with 0.0986MNaOH produced the
Please help! Will Upvote! I'm not sure how to interpret this data!! Thank you 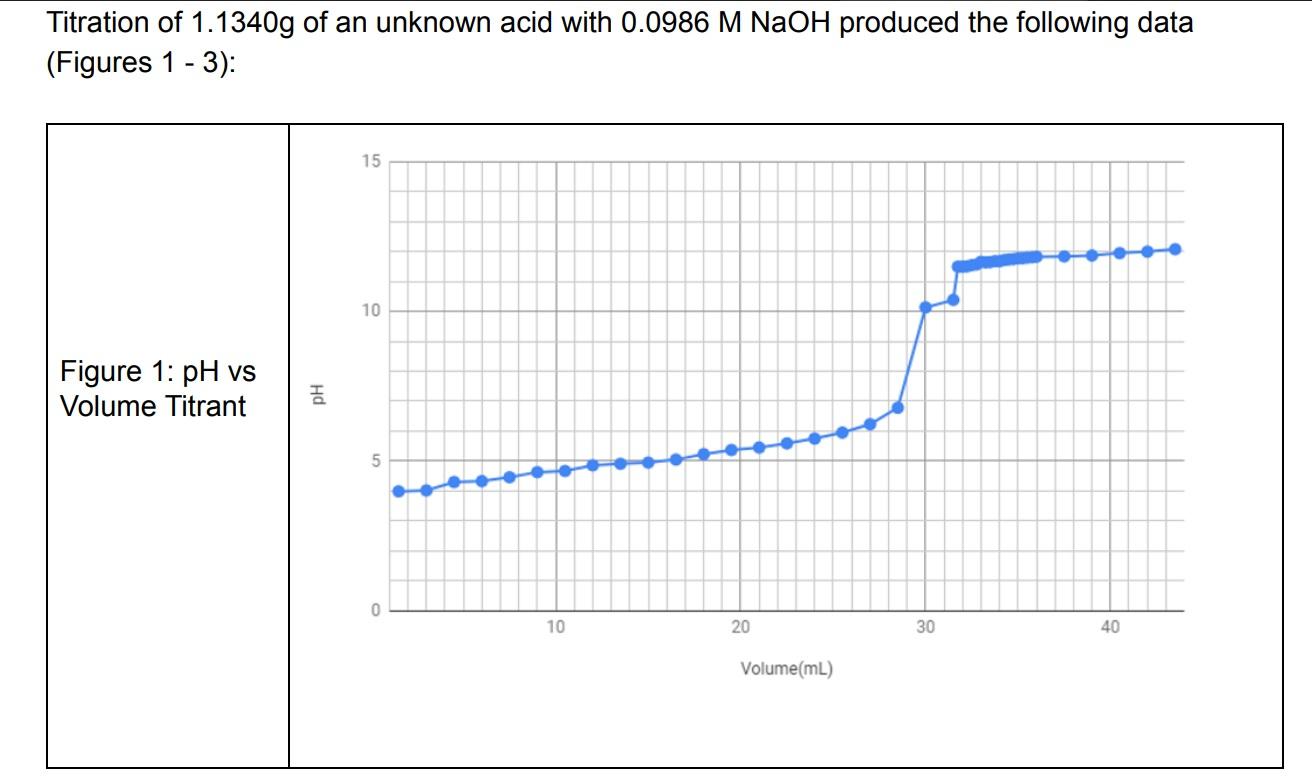
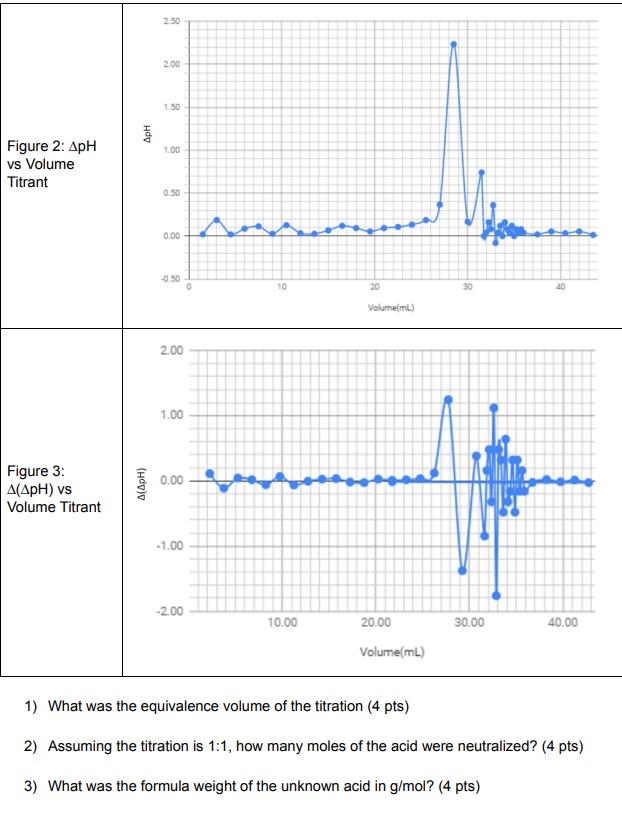
Titration of 1.1340g of an unknown acid with 0.0986MNaOH produced the following data (Figures 1 - 3): 1) What was the equivalence volume of the titration (4 pts) 2) Assuming the titration is 1:1, how many moles of the acid were neutralized? (4 pts) 3) What was the formula weight of the unknown acid in g/mol ? (4 pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


