Question: Please help with a and b. Answers are provided please solve 9. The blue solution formed when an alkali metal dissolves in liquid ammonia consists
Please help with a and b. Answers are provided please solve
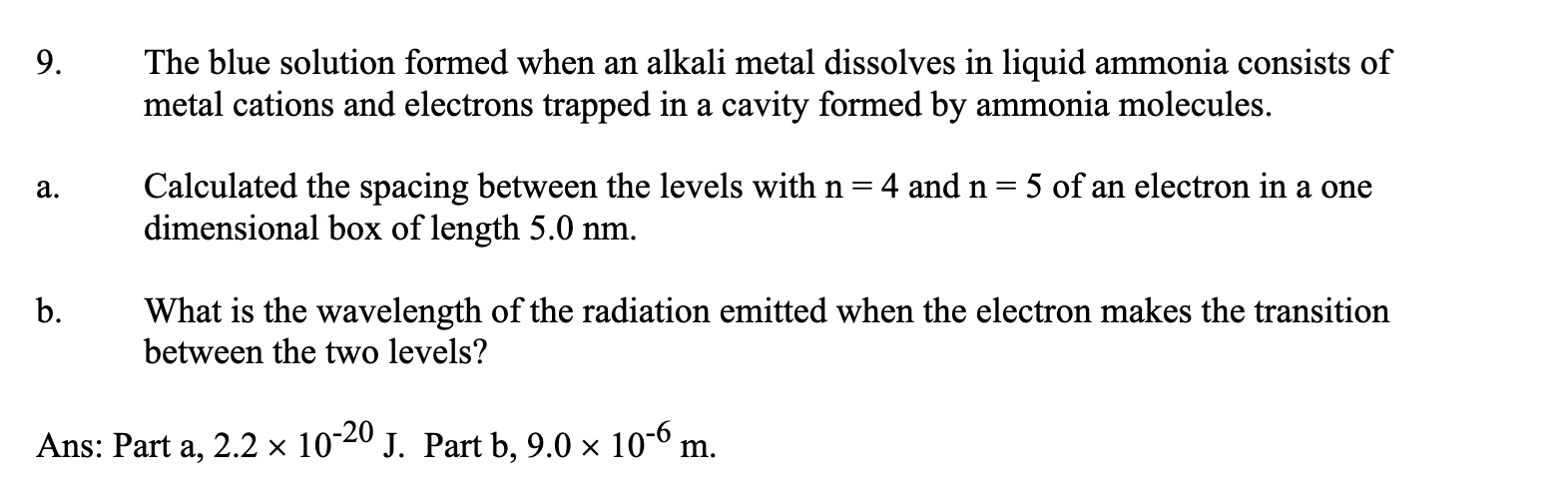
9. The blue solution formed when an alkali metal dissolves in liquid ammonia consists of metal cations and electrons trapped in a cavity formed by ammonia molecules. a. Calculated the spacing between the levels with n=4 and n=5 of an electron in a one dimensional box of length 5.0nm. b. What is the wavelength of the radiation emitted when the electron makes the transition between the two levels? Ans: Part a, 2.21020 J. Part b, 9.0106m
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


