Question: please help with all Question (4 points) a See page: A 0.286 g piece of solid magnesium reacts with gaseous oxygen from the atmosphere to
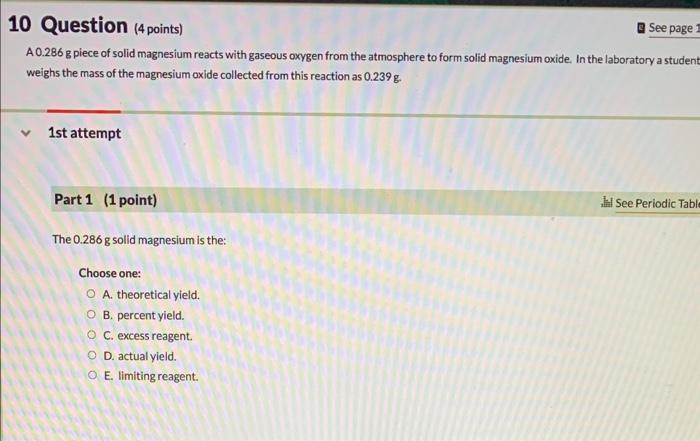
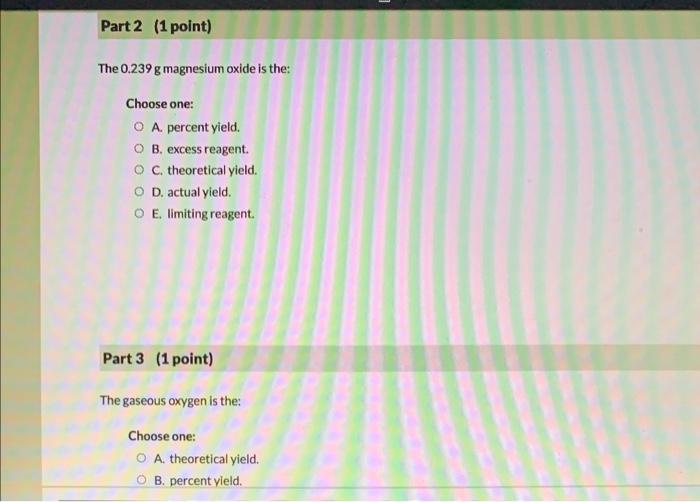
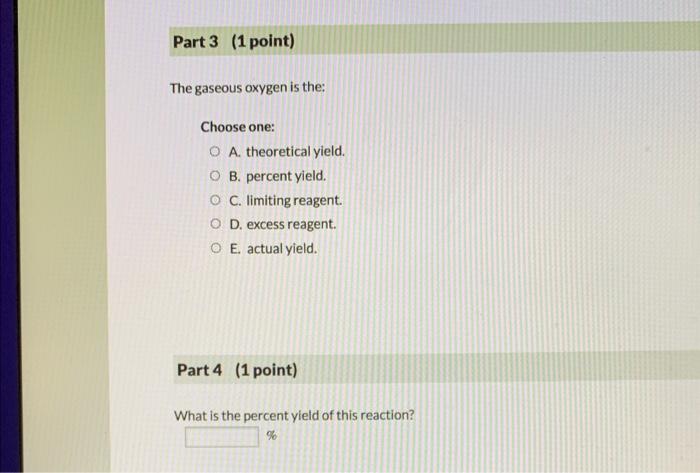
Question (4 points) a See page: A 0.286 g piece of solid magnesium reacts with gaseous oxygen from the atmosphere to form solid magnesium oxide. In the laboratory a studen weighs the mass of the magnesium oxide collected from this reaction as 0.239g. 1st attempt Part 1 (1 point) The 0.286g solid magnesium is the: Choose one: A. theoretical yield. B. percent yield. C. excess reagent. D. actual yield. E. limiting reagent. The 0.239g magnesium oxide is the: Choose one: A. percent yield. B. excess reagent. C. theoretical yield. D. actual yield. E. limiting reagent. Part 3 (1 point) The gaseous oxygen is the: Choose one: A. theoretical yield. B. percent yield. A. theoretical yield. B. percent yield. C. limiting reagent. D. excess reagent. E. actual yield. Part 4 (1 point) What is the percent yield of this reaction? %
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


