Question: please help with this problem. different values than preivous chegg questions. thank you. The heat capacity at constant pressure of a gas is determined experimentally
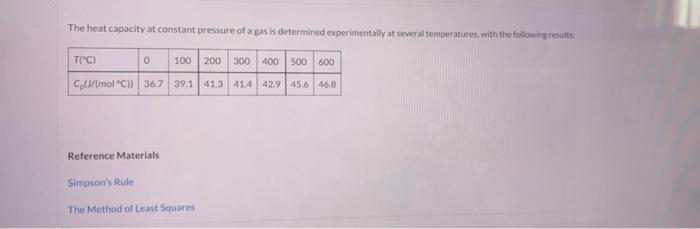
The heat capacity at constant pressure of a gas is determined experimentally at several temperatures with the following results: TI"C) 0 100 200 300 400 500 600 Cu/mol "C)) 367 39.1 413 414 42.9 456 46.8 Reference Materials Simpson's Rule The Method of Least Squares = Simpson's Rule Calculate the heat (kW) required to raise 200.0 mots of the gas from 0C to 600C using Simpson's rule Appendix Alto Integrate the tabulated heat capacities. O= kw e Textbook and Media Save for Late Attempts: 0 of 3 used Sub Integrating a Fitted Line Use the method of least squares (Appendix A.1) to derive a linear expression for CT) in the range O'C to 600C. Use this expression to estimate once again the heat (Wi required to raise 200.0 mols of the gas from 0 to 600C KW
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


