Question: please help with this question. Thank you. (60)The elementary gas reaction AB+C is taking place at constant temperature and pressure in a series of reactors
please help with this question. Thank you.
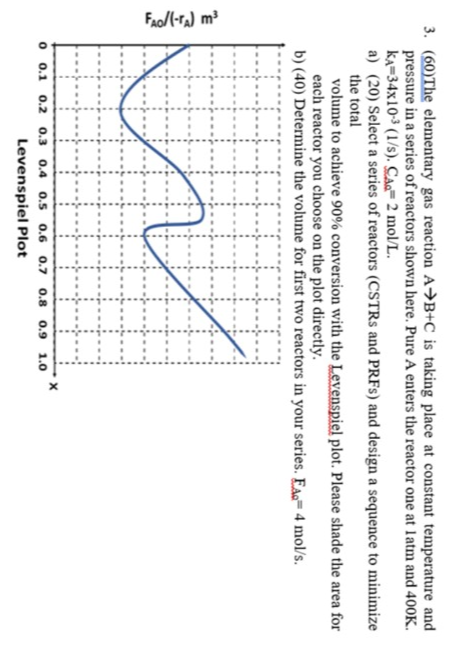
(60)The elementary gas reaction AB+C is taking place at constant temperature and pressure in a series of reactors shown here. Pure A enters the reactor one at 1atm and 400K. kA=34103(1/s).CAlAl=2mol/L. a) (20) Select a series of reactors (CSTRs and PRFs) and design a sequence to minimize the total volume to achieve 90% conversion with the Levenspiel plot. Please shade the area for each reactor you choose on the plot directly. b) (40) Determine the volume for first two reactors in your series. FAd=4mol/s
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


