Question: Please help with this question, thank you! 9. Butane (CH3CH2CH2CH3) can exist in eclipsed and staggered conformations. If butane is viewed down the two center

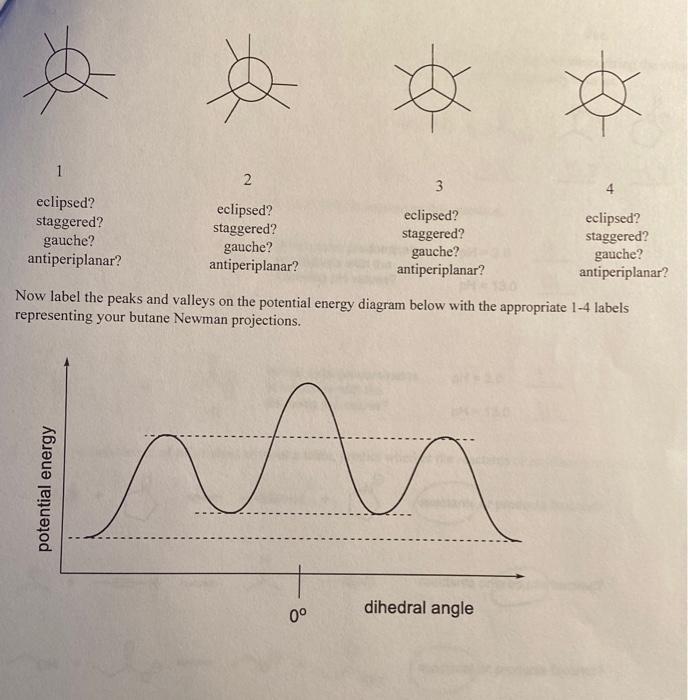
9. Butane (CH3CH2CH2CH3) can exist in eclipsed and staggered conformations. If butane is viewed down the two center carbon atoms, the two methyl groups create two staggered conformations and two eclipsed conformations. Use the templates below and, to each, add 4H-atoms and 2CH3 groups to generate the conformers a-d. 1. Draw the highest energy (least stable) conformer. Circle the appropriate terms below it. 2. Draw the next highest energy conformer. Circle the appropriate terms below it. 3. Draw a gauche conformer. Circle the appropriate terms below it. 4. Draw the lowest energy (most stable) conformer. Circle the appropriate terms below it. 5. One of these should be the anti-periplanar conformer. Which is it? Truw raver une peaks and valleys on the potential energy diagram below with the appropriate 1-4 labels representing your butane Newman projections
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


