Question: Please I already posted the question 3rd time and getting wrong answer. If you are confidence answer it. Other wise don't waste my tooken or
 Please I already posted the question 3rd time and getting wrong answer. If you are confidence answer it. Other wise don't waste my tooken or will get a dislike and feedback!
Please I already posted the question 3rd time and getting wrong answer. If you are confidence answer it. Other wise don't waste my tooken or will get a dislike and feedback!
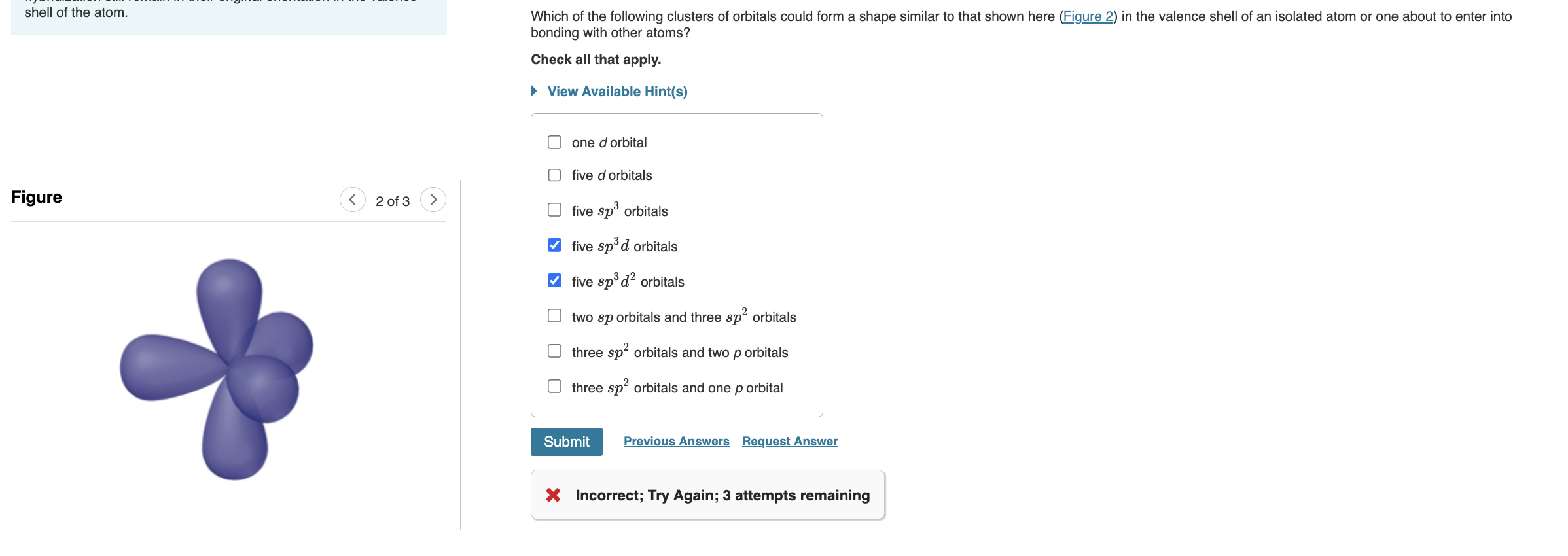
Which of the following clusters of orbitals could form a shape similar to that shown here (Figure 2 ) in the valence shell of an isolated atom or one about to enter into bonding with other atoms? Check all that apply. View Available Hint(s) one d orbital five d orbitals five sp3 orbitals five sp3d orbitals five sp3d2 orbitals two sp orbitals and three sp2 orbitals three sp2 orbitals and two p orbitals three sp2 orbitals and one p orbital 3 Incorrect; Try Again; 3 attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


