Question: Please in short solution Q3. You are given 100 mole of a fuel gas of the following composition, on a mole basis, 20% methane (CH4),
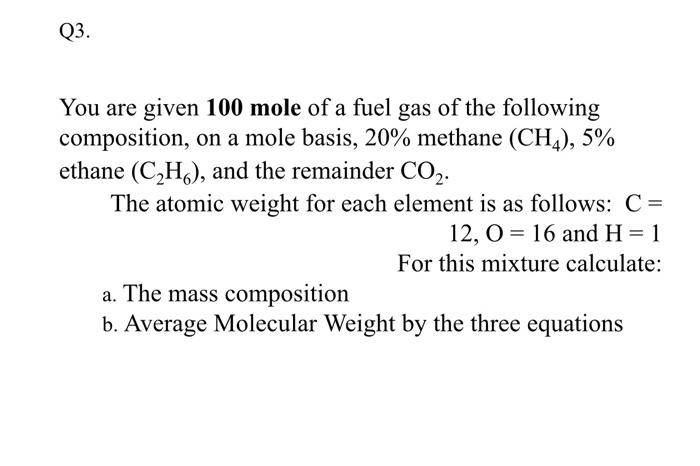
Q3. You are given 100 mole of a fuel gas of the following composition, on a mole basis, 20% methane (CH4), 5% ethane (C2H), and the remainder CO2. The atomic weight for each element is as follows: C= 12,0 = 16 and H= 1 For this mixture calculate: a. The mass composition b. Average Molecular Weight by the three equations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


