Question: PLEASE include all steps Three sequential reactions are occurring in Step 2. Assuming that each reaction produces the zinc (II) ion (Zn2+), write a balanced

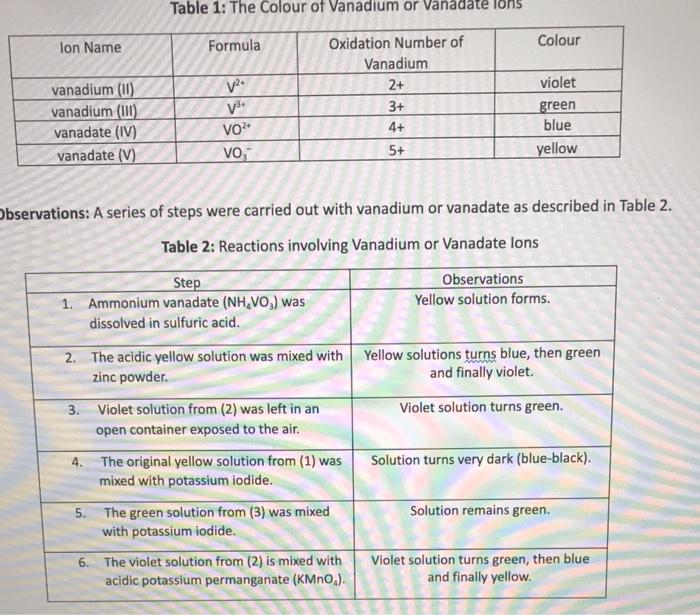
Three sequential reactions are occurring in Step 2. Assuming that each reaction produces the zinc (II) ion (Zn2+), write a balanced redox reaction for the yellow to blue transition (assuming acidic conditions). You can ignore the NH4+and SO42 spectator ions. Remember to add H+ions to balance the charge in an acidic solution if necessary. [T3] 4. When left sitting in Step 3, the vanadium ion reacts with oxygen that is dissolved from the air. Write a balanced redox reaction between the vanadium ion and oxygen. The oxygen is converted to water in this process. Remember to add H+ions to balance the charge in an acidic solution if necessary. [T3] Table 1: The Colour of Vanadium or Vanadate ions servations: A series of steps were carried out with vanadium or vanadate as described in Table Table 2: Reactions involving Vanadium or Vanadate lons
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


