Question: Please include steps and formulas used for calculations as well as explanations. Thank you. 2. Equilibrium constant: CO(g)+H2O(g)CO2(g)+H2(g) has a value of 5102 at a
Please include steps and formulas used for calculations as well as explanations. Thank you.
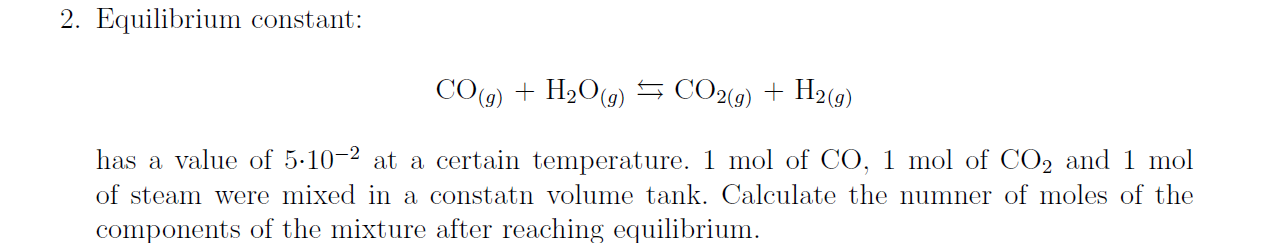
2. Equilibrium constant: CO(g)+H2O(g)CO2(g)+H2(g) has a value of 5102 at a certain temperature. 1mol of CO,1mol of CO2 and 1mol of steam were mixed in a constatn volume tank. Calculate the numner of moles of the components of the mixture after reaching equilibrium
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


