Question: please indicate the answer In a study to determine the rate of the following reaction: 2NO(g)+O2(g)2NO2(g) the concentration of NO was 0.0600Matt=5.0s and 0.0225Matt=650.0s. What
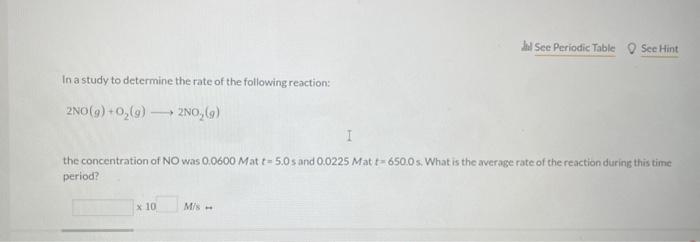
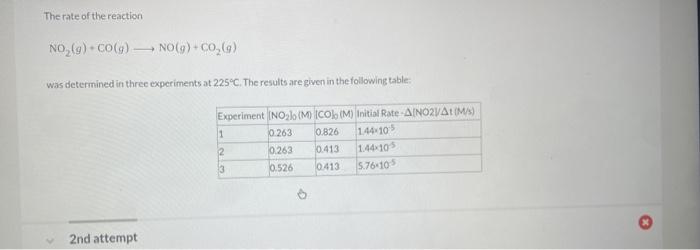
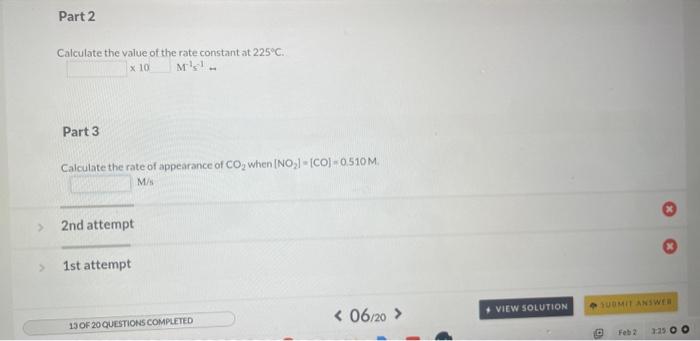
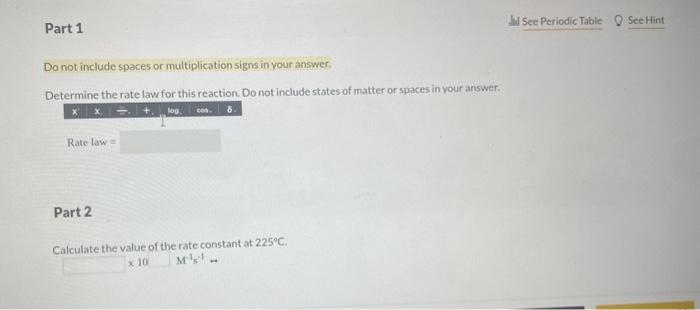
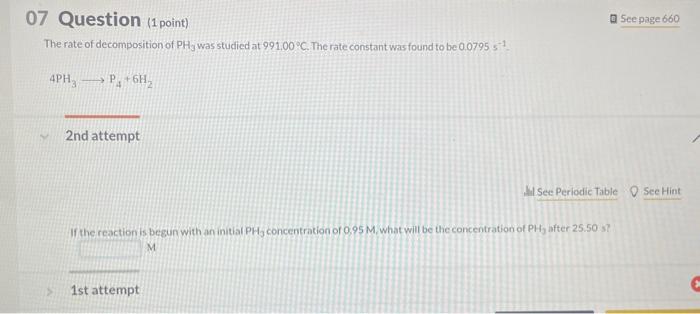
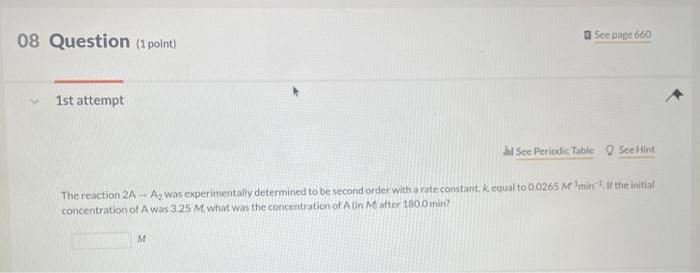
In a study to determine the rate of the following reaction: 2NO(g)+O2(g)2NO2(g) the concentration of NO was 0.0600Matt=5.0s and 0.0225Matt=650.0s. What is the average rate of the reaction durins this time period? The rate of the reaction NO2(g)+CO(g)NO(g)+CO2(q) was determined in three experiments at 225C. The results are given in the following table: Calculate the value of the rate constant at 225C. x. M1s1 - Part 3 Calculate the rate of appearance of CO2 when [NO2]=[CO]=0.510M. M/s 2nd attempt 1st attempt Do not include spaces or multiplication signs in your answer. Determine the rate law for this reaction. Do not include states of matter or spaces in your answer. Part 2 Calculate the value of the rate constant at 225C. 10M1s1 4PH3P4+6H2 2nd attempt If the reaction is begun with an initial PH3 concentration of 0.95M, what will be the concentration of PH3 after 25.50 s? M The reaction 2AA2 was experimentally determined to be second order witha rate constant, k equal to 0.0265M1min1. If the initial concentration of A was 3.25M. what was the concentration of A lin M after 180.0 min? M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


