Question: Using the appropriate thermodynamic values in the table below. calculate the lowest temperature at which the reaction is spontaneous under standard randitim A mixture of
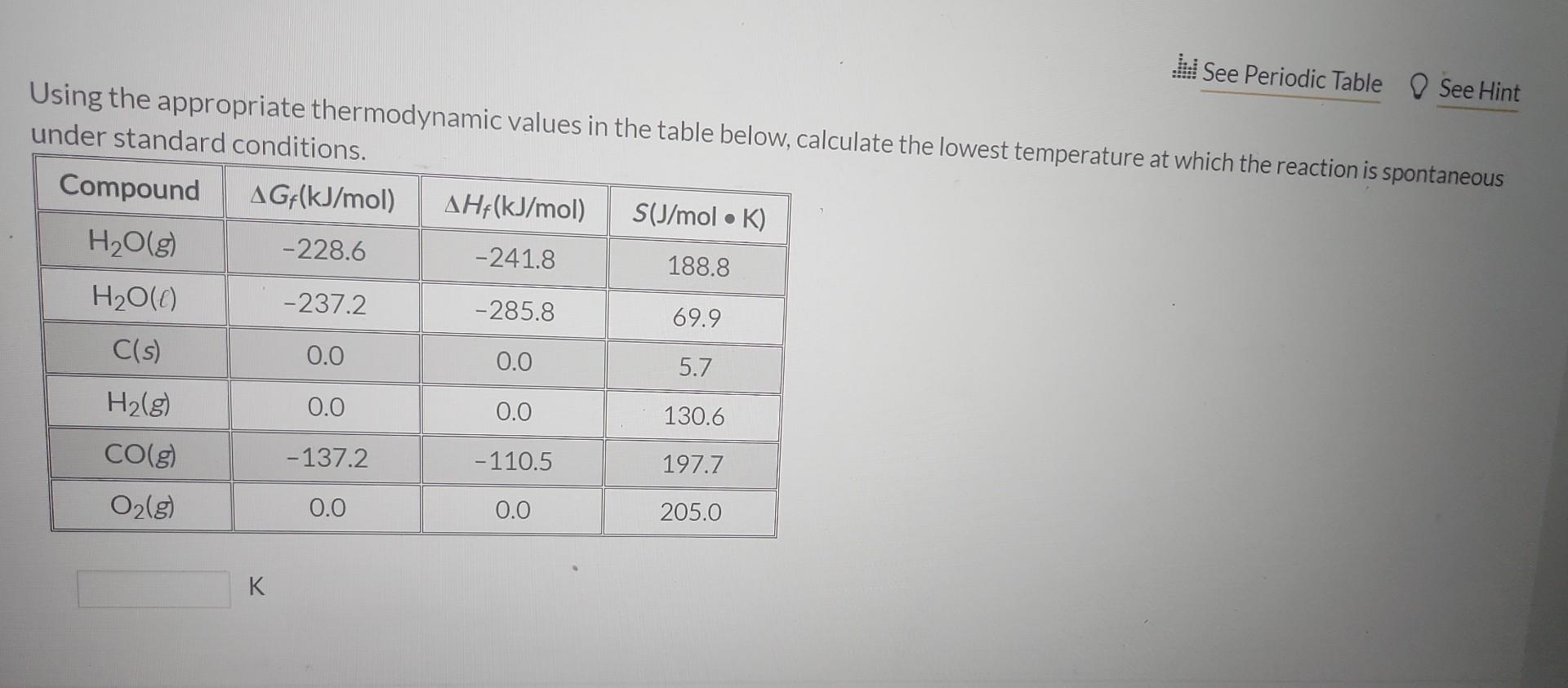
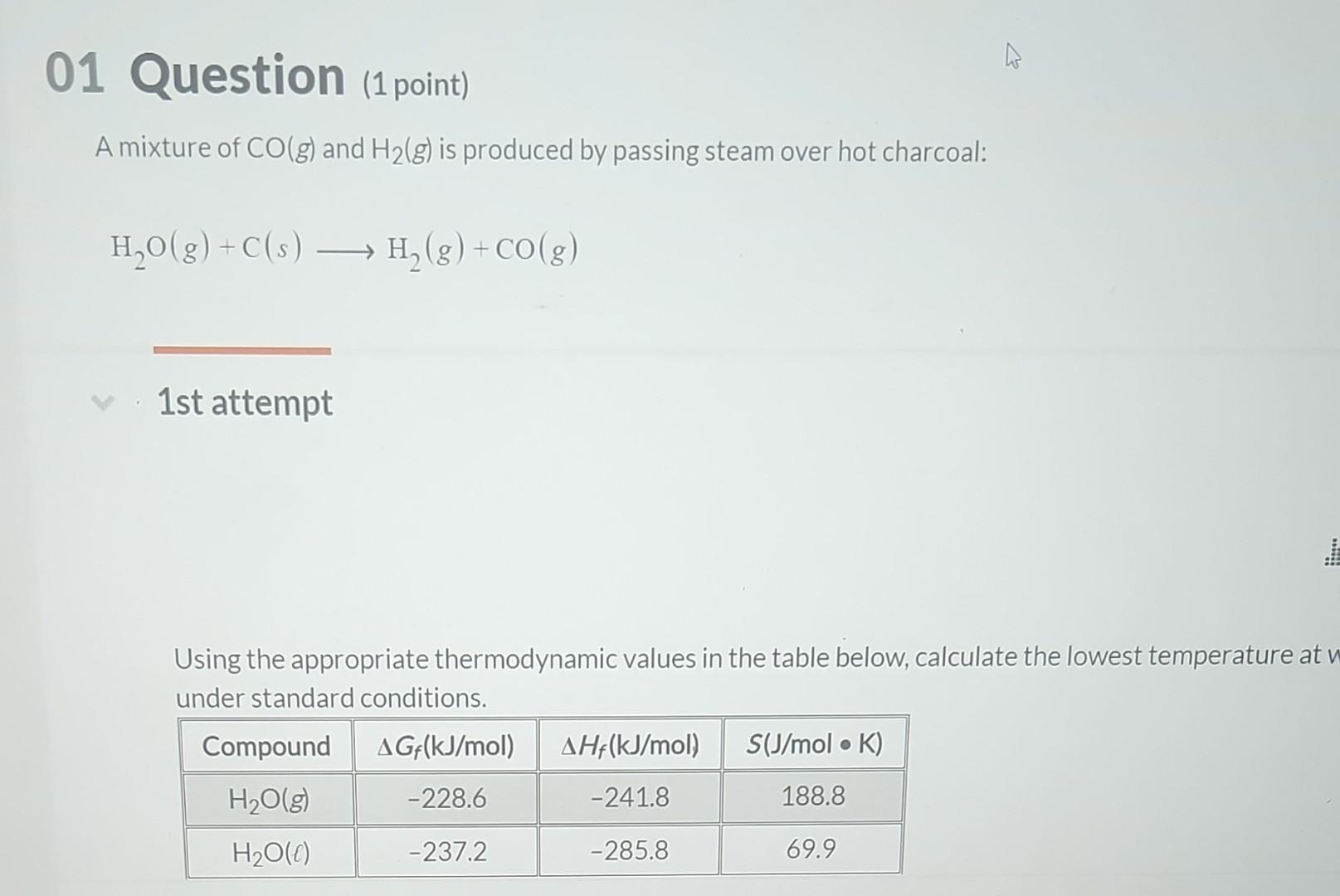
Using the appropriate thermodynamic values in the table below. calculate the lowest temperature at which the reaction is spontaneous under standard randitim A mixture of CO(g) and H2(g) is produced by passing steam over hot charcoal: H2O(g)+C(s)H2(g)+CO(g) 1st attempt Using the appropriate thermodynamic values in the table below, calculate the lowest temperature at under standard conditions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


