Question: Please note that geometry refers to the molecular or ionic geometry. F -B-F: A. The Lewis diagram for BF3 is: 1 F: The electron-pair geometry
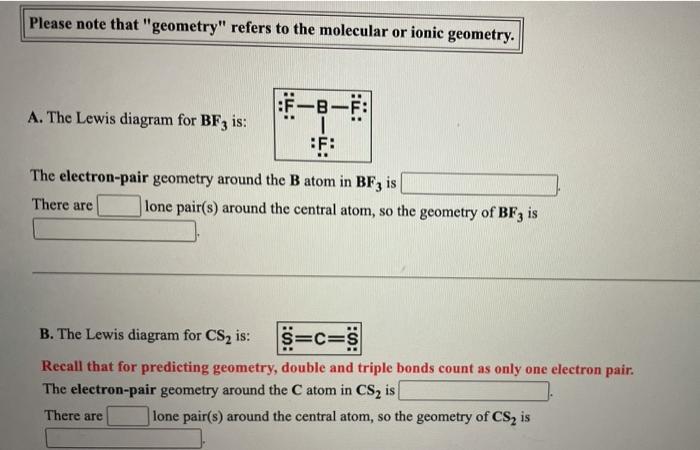
Please note that "geometry" refers to the molecular or ionic geometry. F -B-F: A. The Lewis diagram for BF3 is: 1 F: The electron-pair geometry around the Batom in BF3 is There are lone pair(s) around the central atom, so the geometry of BF3 is B. The Lewis diagram for CS2 is: S=C=S Recall that for predicting geometry, double and triple bonds count as only one electron pair. The electron-pair geometry around the C atom in CS2 is There are lone pair(s) around the central atom, so the geometry of CS, is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


