Question: Balance the following redox reaction and type the coefficients for each of the species in the balanced reaction. 2Al(s) + 3 F (g) 2Al+
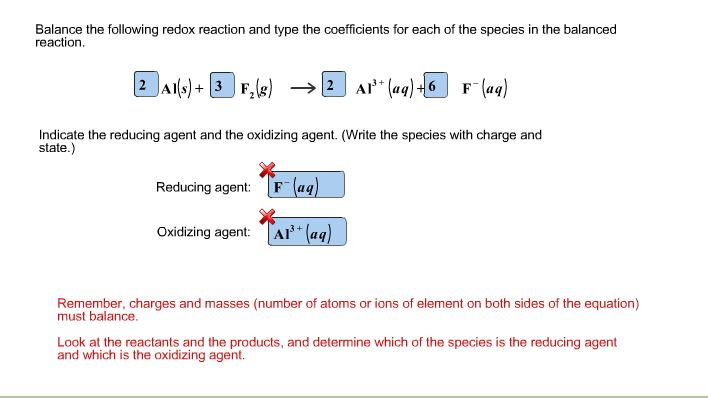
Balance the following redox reaction and type the coefficients for each of the species in the balanced reaction. 2Al(s) + 3 F (g) 2Al+ (aq) +6 F- (aq) Indicate the reducing agent and the oxidizing agent. (Write the species with charge and state.) Reducing agent: F (aq) TAP+ (aq) Oxidizing agent: Remember, charges and masses (number of atoms or ions of element on both sides of the equation) must balance. Look at the reactants and the products, and determine which of the species is the reducing agent and which is the oxidizing agent.
Step by Step Solution
3.50 Rating (150 Votes )
There are 3 Steps involved in it
Balanced chemical equation 2 AIF 2 Als 3 Fg 3... View full answer

Get step-by-step solutions from verified subject matter experts


