Question: Please note that the final answers are in RED. Please verify that you got the same answer. Thanks A+BC follows an elementary rate law and
Please note that the final answers are in RED. Please verify that you got the same answer. Thanks
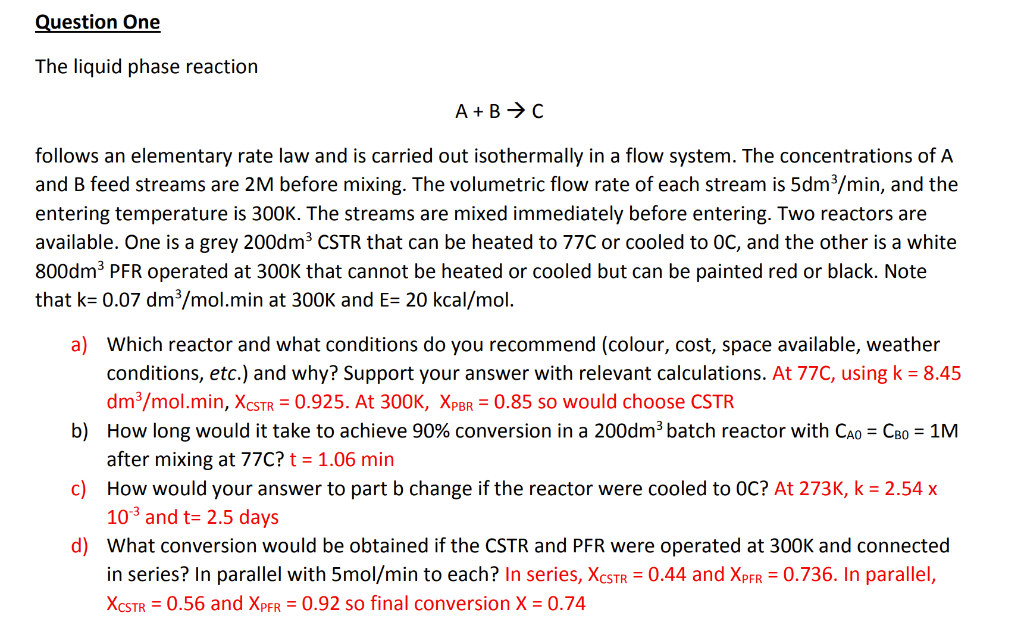
A+BC follows an elementary rate law and is carried out isothermally in a flow system. The concentrations of A and B feed streams are 2M before mixing. The volumetric flow rate of each stream is 5dm3/min, and the entering temperature is 300K. The streams are mixed immediately before entering. Two reactors are available. One is a grey 200dm3 CSTR that can be heated to 77C or cooled to 0C, and the other is a white 800dm3 PFR operated at 300K that cannot be heated or cooled but can be painted red or black. Note that k=0.07dm3/mol.min at 300K and E=20kcal/mol. a) Which reactor and what conditions do you recommend (colour, cost, space available, weather conditions, etc.) and why? Support your answer with relevant calculations. At 77C, using k=8.45 dm3/mol.min,XCSTR=0.925. At 300K, XPBR=0.85 so would choose CSTR b) How long would it take to achieve 90% conversion in a 200dm3 batch reactor with CA0=CB0=1M after mixing at 77C ? t=1.06min c) How would your answer to part b change if the reactor were cooled to 0C ? At 273K,k=2.54x 103 and t=2.5 days d) What conversion would be obtained if the CSTR and PFR were operated at 300K and connected in series? In parallel with 5mol/min to each? In series, XCSTR=0.44 and XPFR=0.736. In parallel, XCSTR=0.56 and XPFR=0.92 so final conversion X=0.74
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


