Question: Please notes the Q asking about Kc not Kp 0. When a sample of 0.640 mole of NOCl was placed in a 1.00L container at
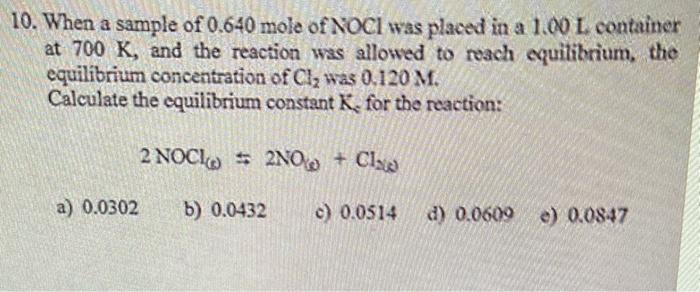
0. When a sample of 0.640 mole of NOCl was placed in a 1.00L container at 700K, and the reaction was allowed to reach equilibrium, the equilibrium concentration of Cl2 was 0.120M. Calculate the equilibrium constant K, for the reaction: 2NOCl()2NO+Cl2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


