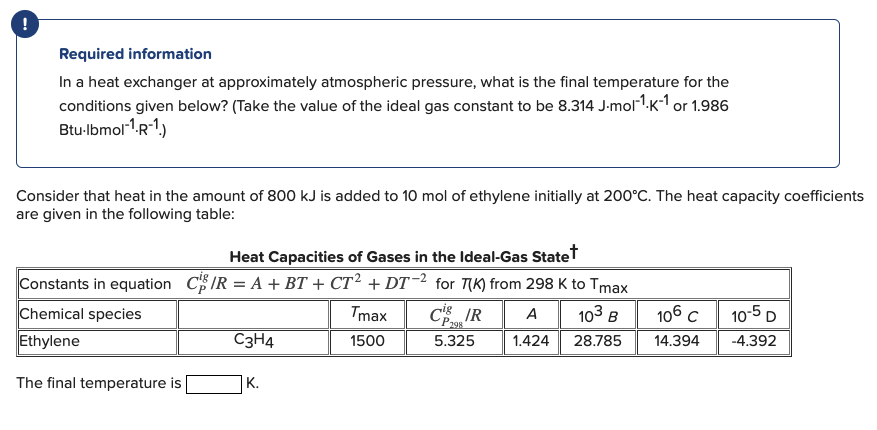
Question: PLEASE ONLY ANSWER THIS QUESTION DON'T PASTE A ANSWER TO A SIMILAR QUESTION WILL UPVOTE!!! ! Required information In a heat exchanger at approximately atmospheric
PLEASE ONLY ANSWER THIS QUESTION DON'T PASTE A ANSWER TO A SIMILAR QUESTION
WILL UPVOTE!!!
! Required information In a heat exchanger at approximately atmospheric pressure, what is the final temperature for the conditions given below? (Take the value of the ideal gas constant to be 8.314 J.mol-?.K-1 or 1.986 Btu-Ibmoll.r-?) Consider that heat in the amount of 800 kJ is added to 10 mol of ethylene initially at 200C. The heat capacity coefficients are given in the following table: Heat Capacities of Gases in the Ideal-Gas Statet Constants in equation CS/R = A + BT + CT2 + DT-2 for Tik) from 298 K to Tmax Chemical species Tmax COS IR A Ethylene C3H4 1500 5.325 1.424 28.785 103 B 106c 14.394 10-5D -4.392 The final temperature is K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


