Question: Please present answer in equation form like in the picture This question will walk you through the steps of determining which reactant is limiting based
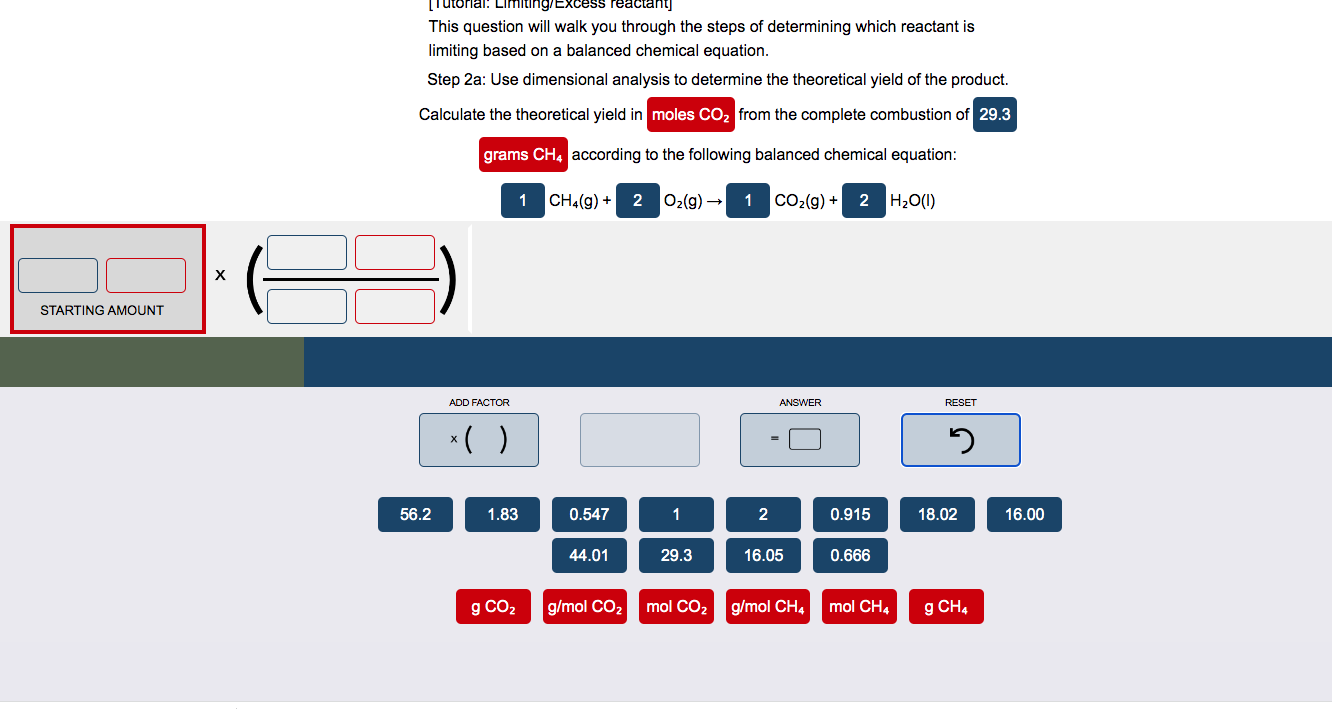 Please present answer in equation form like in the picture
Please present answer in equation form like in the picture
This question will walk you through the steps of determining which reactant is limiting based on a balanced chemical equation. Step 2a: Use dimensional analysis to determine the theoretical yield of the product. Calculate the theoretical yield in from the complete combustion of according to the following balanced chemical equation: CH4(g)+O2(g)CO2(g)+H2O(I)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


