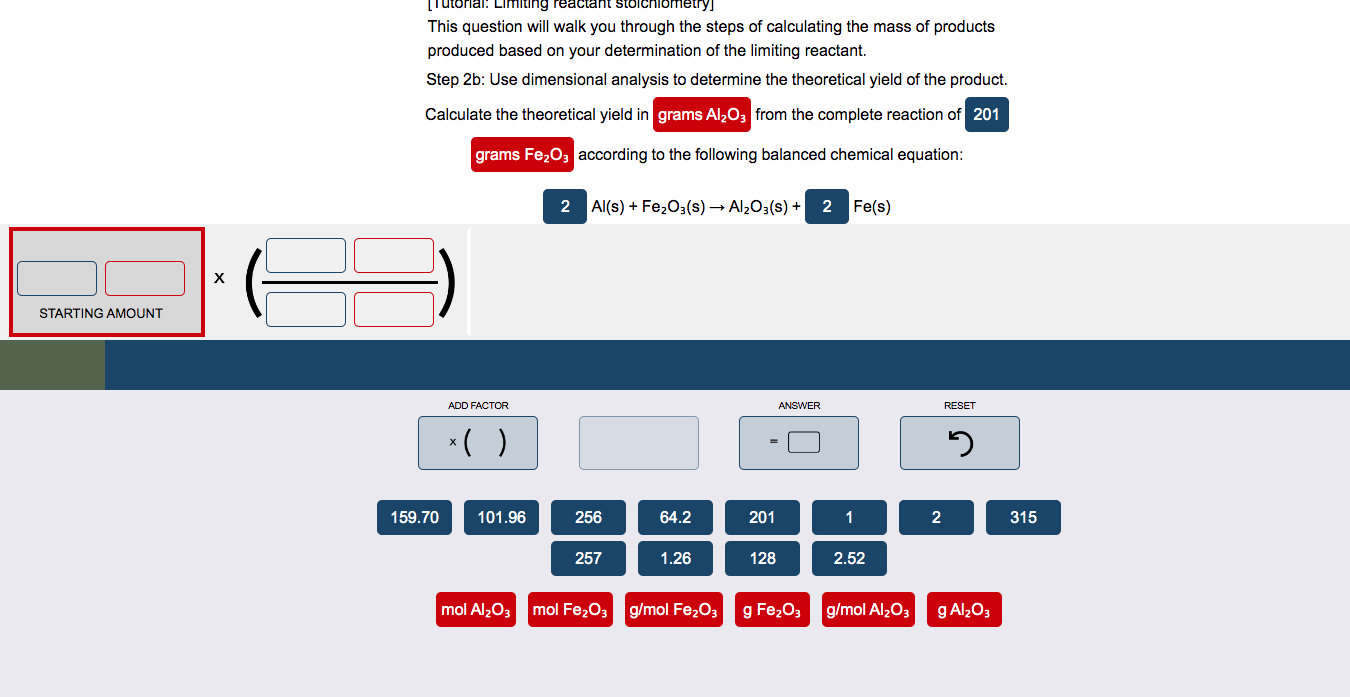
Question: Please present the answer as displayed in the image (in a equation) This question will walk you through the steps of calculating the mass of
 Please present the answer as displayed in the image (in a equation)
Please present the answer as displayed in the image (in a equation)
This question will walk you through the steps of calculating the mass of products produced based on your determination of the limiting reactant. Step 2b : Use dimensional analysis to determine the theoretical yield of the product. Calculate the theoretical yield in from the complete reaction of according to the following balanced chemical equation: Al(s)+Fe2O3(s)Al2O3(s)+Fe(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


