Question: PLEASE READ: Attached is a part a , b , c of a question, the first drop down menu option = the things that are
PLEASE READ: Attached is a part ab c of a question, the first drop down menu option the things that are to the right of the questions, the one below part C is the one that would be the second option for a drop down for all parts. All info is provided.
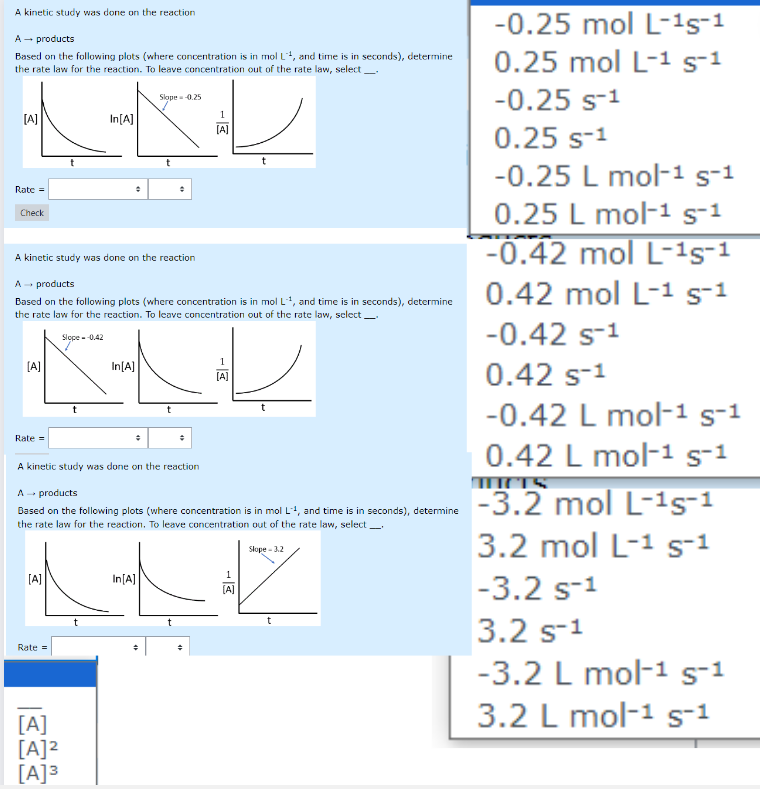
A kinetic study was done on the reaction
products
Based on the following plots where concentration is in and time is in seconds determine
the rate law for the reaction. To leave concentration out of the rate law, select
Rate
A kinetic study was done on the reaction
products
Based on the following plots where concentration is in and time is in seconds determine
the rate law for the reaction. To leave concentration out of the rate law, select
Rate
A kinetic study was done on the reaction
products
Based on the following plots where concentration is in and time is in seconds determine
the rate law for the reaction. To leave concentration out of the rate law, select
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


