Question: please see if question 1 is correct and help with all the other questions. thank you uestions 1. Use the mass of the unknown amino


![acid (to the nearest whole number). [Given. g=MLfw ] MM1=g/MV=19/M0.011=10.001WfU=V/10.001 2. Use](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8efaab0073_31466f8efaa4078d.jpg)
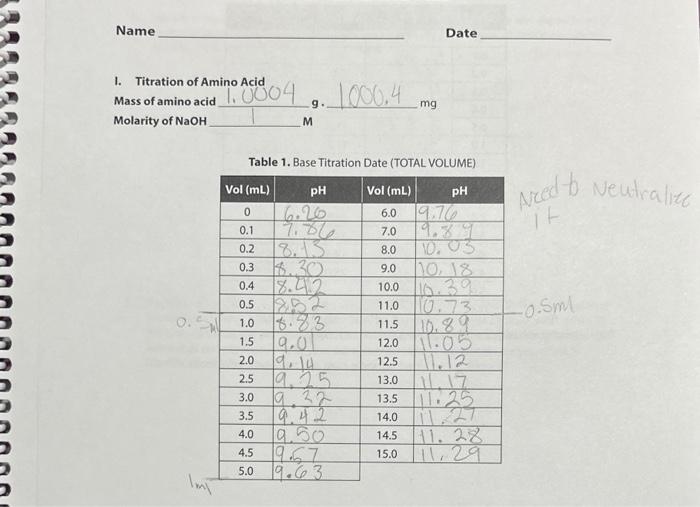
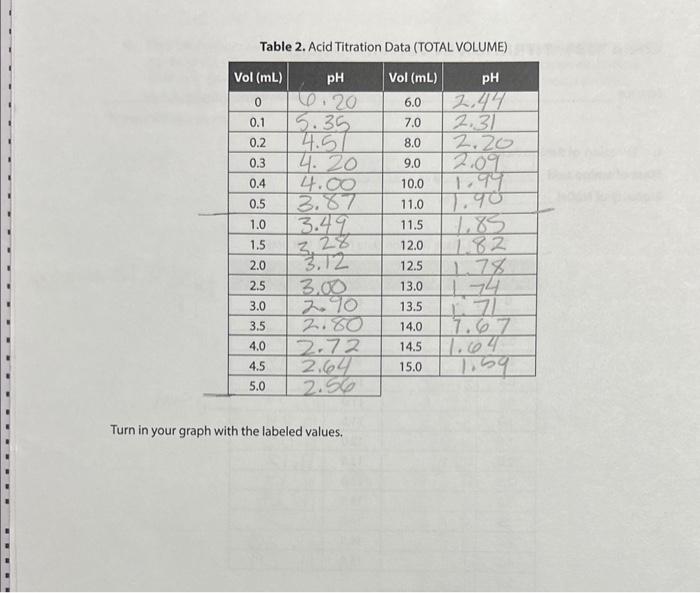
uestions 1. Use the mass of the unknown amino acid, the equivalence point volume and the molarity of the NaOH to calculate the formula weight (also called the molar mass) of amino acid (to the nearest whole number). [Given. g=MLfw ] MM1=g/MV=19/M0.011=10.001WfU=V/10.001 2. Use your values of pKa and pK22 and molecular weight to identity your amino acid as one of the twenty shown on Table 1. Compare (discuss the closeness) your experimental values to the ones of the amino acid you select. Use the values on the table to calculate an expected value of pl and compare that to your experimental value. How certain are you of your identification? Why would just two pKa's alone probably be insufficient? 3. Sketch (no numbers) a general titration curve for your amino acid below (see Introduction, p 3). Place a label " 1 " near the very lowest pH, a label "I" in the center fat the pl), and add a label "7II" at the very highest pH. For each of the three regions 1,11 , and III, draw in the expected structural form your amino acid. 4. In the curve above, what kind of solution is represented by the flat regions? Go back and write labels " B1" and " B2 on the sketch. a. What are the structures of the two chemical species present at B1? b. What are the structures of the two chemical species present at B2? 1. Titration of Amino Acid Mass of amino acid g. mg Molarity of NaOH M Table 1. Base Titration Date (TOTAL VOLUME) Table 2. Acid Titration Data (TOTAL VOLUME) Turn in your graph with the labeled values. Table 1. Constants for Amino Acids PROCEDURES 1. Titration of amino acid 1. Weigh out about 1.000g of amino acid, record the exact mass in g and mg and transfer to a 100 -mL graduated cylinder, fill about half full with distilled water and swirl to dissolve. If you stir with a stirring rod, rinse your stirring rod into the solution to avold taking any of the amino acid away, it should dissolve readily. When it is all dissolved, make up to volume (100 mm.). 2. Transfer the amino acid solution to a clean 250 -mL beakec, rinse the graduated cylinder slightly into the beaker to transfer all the amino acid, add a small stir bar, set on the stirring plate, and insert the pH meter so that it is not likely to be struck by the stir bar, 3. Allow a few seconds for the meter to stabilize and record the initial pH. (The pH meter's electronic response may be slow, so it is important that you wait for the display to stop changing and say "ready," This may take 30 seconds or more) 4. You will first titrate the amino acid with strong base, 1MNaOH. Record the exact molarity of: the base. Add the suggested volumes of base, wait for the pit meter to respond, and record 34 uestions 1. Use the mass of the unknown amino acid, the equivalence point volume and the molarity of the NaOH to calculate the formula weight (also called the molar mass) of amino acid (to the nearest whole number). [Given. g=MLfw ] MM1=g/MV=19/M0.011=10.001WfU=V/10.001 2. Use your values of pKa and pK22 and molecular weight to identity your amino acid as one of the twenty shown on Table 1. Compare (discuss the closeness) your experimental values to the ones of the amino acid you select. Use the values on the table to calculate an expected value of pl and compare that to your experimental value. How certain are you of your identification? Why would just two pKa's alone probably be insufficient? 3. Sketch (no numbers) a general titration curve for your amino acid below (see Introduction, p 3). Place a label " 1 " near the very lowest pH, a label "I" in the center fat the pl), and add a label "7II" at the very highest pH. For each of the three regions 1,11 , and III, draw in the expected structural form your amino acid. 4. In the curve above, what kind of solution is represented by the flat regions? Go back and write labels " B1" and " B2 on the sketch. a. What are the structures of the two chemical species present at B1? b. What are the structures of the two chemical species present at B2? 1. Titration of Amino Acid Mass of amino acid g. mg Molarity of NaOH M Table 1. Base Titration Date (TOTAL VOLUME) Table 2. Acid Titration Data (TOTAL VOLUME) Turn in your graph with the labeled values. Table 1. Constants for Amino Acids PROCEDURES 1. Titration of amino acid 1. Weigh out about 1.000g of amino acid, record the exact mass in g and mg and transfer to a 100 -mL graduated cylinder, fill about half full with distilled water and swirl to dissolve. If you stir with a stirring rod, rinse your stirring rod into the solution to avold taking any of the amino acid away, it should dissolve readily. When it is all dissolved, make up to volume (100 mm.). 2. Transfer the amino acid solution to a clean 250 -mL beakec, rinse the graduated cylinder slightly into the beaker to transfer all the amino acid, add a small stir bar, set on the stirring plate, and insert the pH meter so that it is not likely to be struck by the stir bar, 3. Allow a few seconds for the meter to stabilize and record the initial pH. (The pH meter's electronic response may be slow, so it is important that you wait for the display to stop changing and say "ready," This may take 30 seconds or more) 4. You will first titrate the amino acid with strong base, 1MNaOH. Record the exact molarity of: the base. Add the suggested volumes of base, wait for the pit meter to respond, and record 34
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


