Question: Please show all work. Hexane is fed at a rate of 13.53 mol/s and burned with oxygen (fed at 115.005 mol/s) so that four moles

Please show all work.
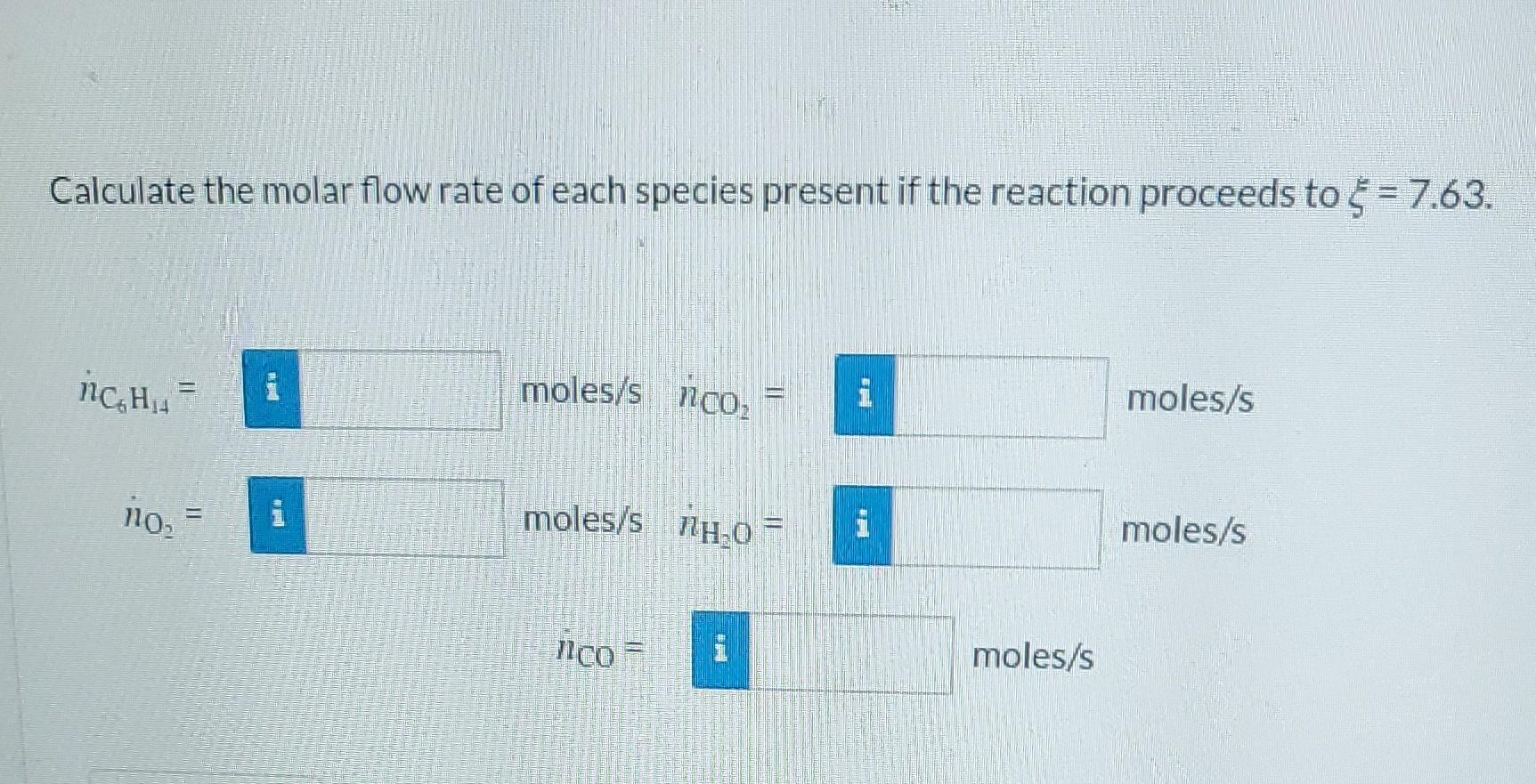
Hexane is fed at a rate of 13.53 mol/s and burned with oxygen (fed at 115.005 mol/s) so that four moles of CO are produced for every mole of hexane according to the reaction stoichiometry CoHis+AOz - 4CO+BCO,+CH,O Determine the values of A, B, and C in the reaction above for the hexane coefficient having a value of 1 mole. They may be decimal representations of rational numbers (i.e. 3/2 = 1.5). A = i moles B = i moles Ci moles Calculate the molar flow rate of each species present if the reaction proceeds to = 7.63. nc H moles/s nco moles/s LI Calculate the molar flow rate of each species present if the reaction proceeds to = 7.63. moles/s nco 1 moles/s ncH= moles/s H0 moles/s no, = CO i moles/s
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


