Question: Please show all work. Thank you. = 2. a) The fractionation factor for oxygen in water at 25C is a, = 1.0092 and for hydrogen
Please show all work. Thank you.
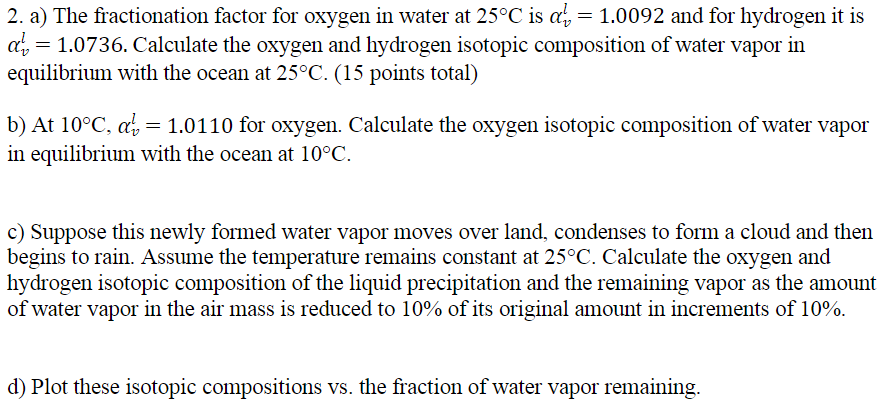
= 2. a) The fractionation factor for oxygen in water at 25C is a, = 1.0092 and for hydrogen it is a', = 1.0736. Calculate the oxygen and hydrogen isotopic composition of water vapor in equilibrium with the ocean at 25C. (15 points total) = b) At 10C, a', = 1.0110 for oxygen. Calculate the oxygen isotopic composition of water vapor in equilibrium with the ocean at 10C. c) Suppose this newly formed water vapor moves over land, condenses to form a cloud and then begins to rain. Assume the temperature remains constant at 25C. Calculate the oxygen and hydrogen isotopic composition of the liquid precipitation and the remaining vapor as the amount of water vapor in the air mass is reduced to 10% of its original amount in increments of 10%. d) Plot these isotopic compositions vs. the fraction of water vapor remaining. = 2. a) The fractionation factor for oxygen in water at 25C is a, = 1.0092 and for hydrogen it is a', = 1.0736. Calculate the oxygen and hydrogen isotopic composition of water vapor in equilibrium with the ocean at 25C. (15 points total) = b) At 10C, a', = 1.0110 for oxygen. Calculate the oxygen isotopic composition of water vapor in equilibrium with the ocean at 10C. c) Suppose this newly formed water vapor moves over land, condenses to form a cloud and then begins to rain. Assume the temperature remains constant at 25C. Calculate the oxygen and hydrogen isotopic composition of the liquid precipitation and the remaining vapor as the amount of water vapor in the air mass is reduced to 10% of its original amount in increments of 10%. d) Plot these isotopic compositions vs. the fraction of water vapor remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


