Question: PLEASE SHOW ALL WORK!!Thank you so much! 5. A sealed 24.0-m3 tank is filled with 1,423 moles of oxygen gas (O2) at an initial temperature
PLEASE SHOW ALL WORK!!Thank you so much!
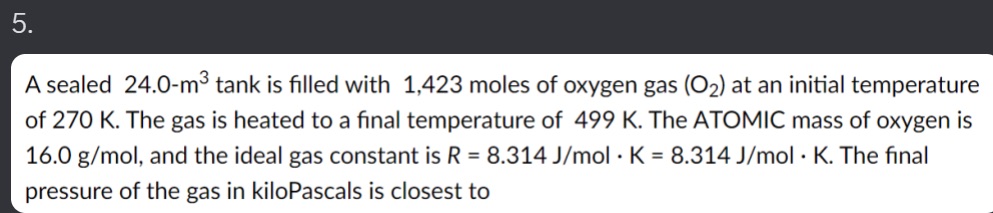
5. A sealed 24.0-m3 tank is filled with 1,423 moles of oxygen gas (O2) at an initial temperature of 270 K. The gas is heated to a final temperature of 499 K. The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol . K = 8.314 J/mol . K. The final pressure of the gas in kiloPascals is closest to
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


