Question: Please show how to work through this problem type so that I may apply it towards other questions similar to this. Thank you! When one
Please show how to work through this problem type so that I may apply it towards other questions similar to this. Thank you!
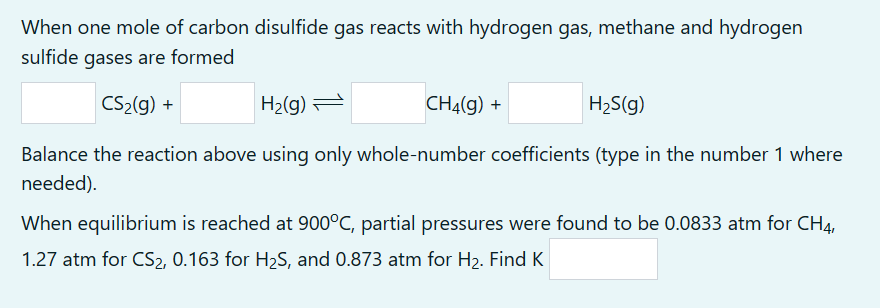
When one mole of carbon disulfide gas reacts with hydrogen gas, methane and hydrogen sulfide gases are formed CS2(g)+H2(g)CH4(g)+H2S(g) Balance the reaction above using only whole-number coefficients (type in the number 1 where needed). When equilibrium is reached at 900C, partial pressures were found to be 0.0833atm for CH4, 1.27 atm for CS2,0.163 for H2S, and 0.873 atm for H2. Find K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


