Question: please show matlab code (the entire function, the variables, etc) Find the specific volume v (m^3/kg) of methane (CH_4) at p = 8 MPa, T
please show matlab code (the entire function, the variables, etc)
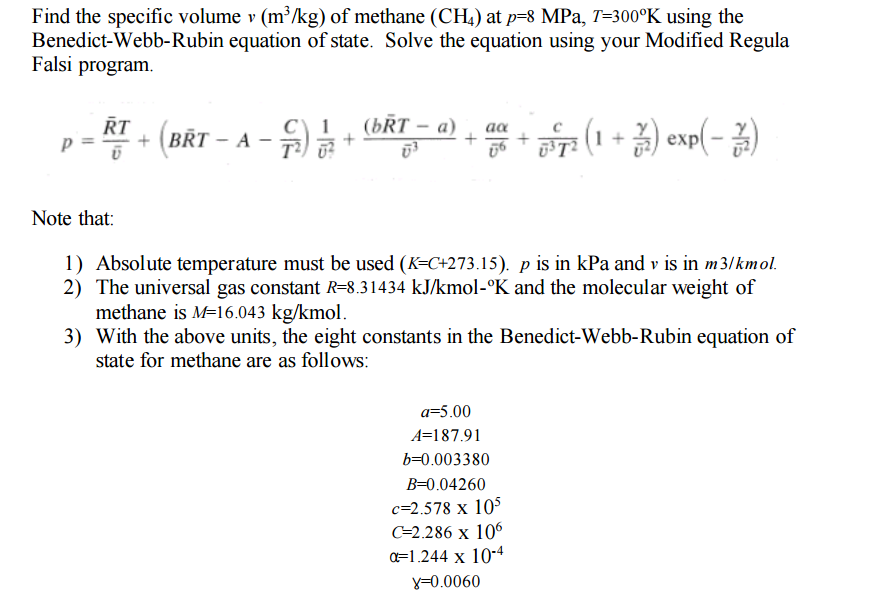
Find the specific volume v (m^3/kg) of methane (CH_4) at p = 8 MPa, T = 300 degree K using the Benedict-Webb-Rubin equation of state. Solve the equation using your Modified Regula Falsi program. p = RT/v + (BRT - A - C/T^2) 1/v^2 + (bRT - a)/v^3 + a alpha/v^6 + c/v^3 T^2 (1 + gamma/v^2) exp(-gamma/v^2) Note that: 1) Absolute temperature must be used (K = C + 273.15). p is in kPa and v is in m3/kmol. 2) The universal gas constant R = 8.31434 kJ/kmol- degree K and the molecular weight of methane is M = 16.043 kg/kmol. 3) With the above units, the eight constants in the Benedict-Webb-Rubin equation of state for methane are as follows: a = 5.00 A = 187.91 b = 0.003380 B = 0.04260 c = 2.578 times 10^5 C = 2.286 times 10^6 alpha = 1.244 times 10^-4 gamma = 0.0060
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


