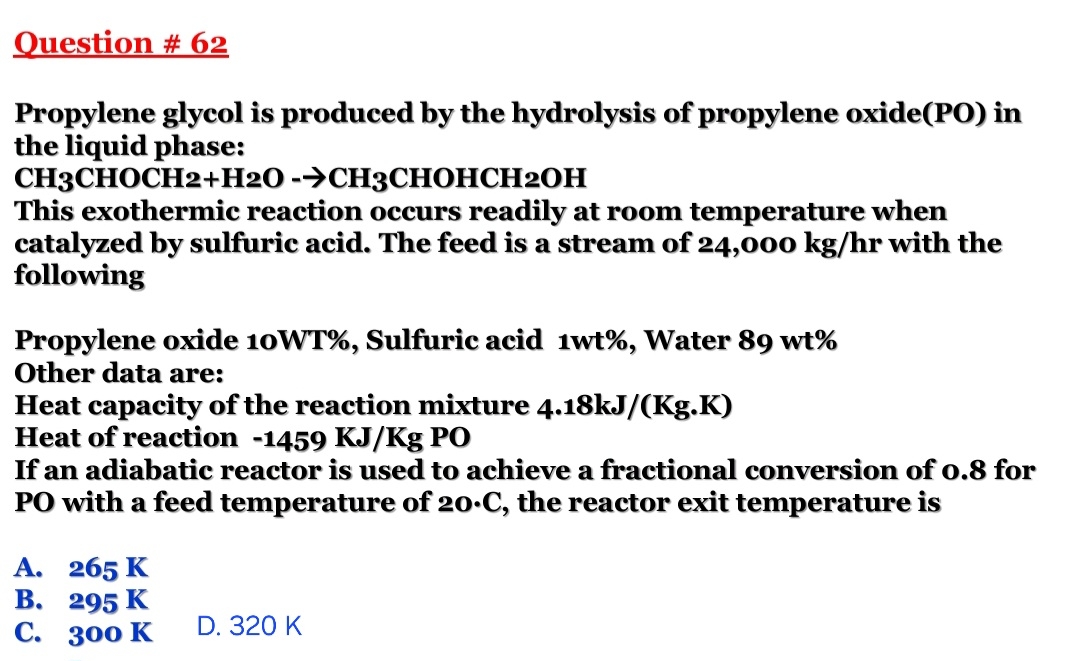
Question: Please show steps and Explanation Question # 6 2 Propylene glycol is produced by the hydrolysis of propylene oxide ( PO ) in the liquid
Please show steps and Explanation
Question #
Propylene glycol is produced by the hydrolysis of propylene oxidePO in the liquid phase:
CHOCHCHOHCH
This exothermic reaction occurs readily at room temperature when catalyzed by sulfuric acid. The feed is a stream of with the following
Propylene oxide WT Sulfuric acid Water Other data are:
Heat capacity of the reaction mixture
Heat of reaction PO
If an adiabatic reactor is used to achieve a fractional conversion of for PO with a feed temperature of the reactor exit temperature is
A
B
C
D
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


