Question: please show workings!! imp Given the following balanced equation for the combustion of liquid undecane: C11H24(l)+34O2(g)11CO2(g)+12H2O(g) Suppose 0.403 moles of H2O is produced after a
please show workings!! imp 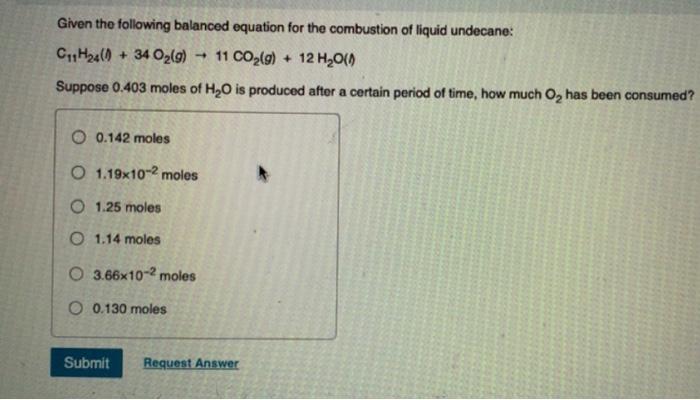

Given the following balanced equation for the combustion of liquid undecane: C11H24(l)+34O2(g)11CO2(g)+12H2O(g) Suppose 0.403 moles of H2O is produced after a certain period of time, how much O2 has been consumed? 0.142 moles 1.19102 moles 1.25 moles 1.14 moles 3.66102 moles 0.130 moles
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


