Question: Please solve 1 a and b 1. ) The half-life period for a certain first order reaction is 45.5 minutes. How long will it take
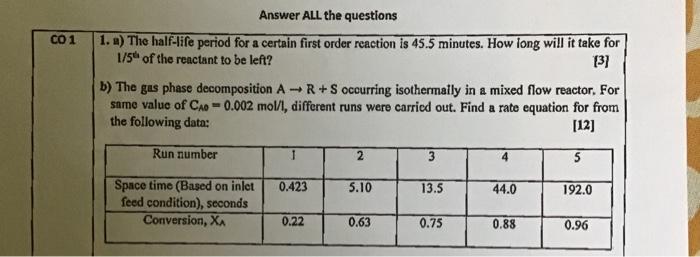
1. ) The half-life period for a certain first order reaction is 45.5 minutes. How long will it take for 1/5t of the reactant to be left? [3] b) The gas phase decomposition AR+S occurring isothermally in a mixed flow reactor. For same value of CAO=0.002mol/l, different runs were carricd out. Find a rate equation for from the following data: [12]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


