Question: please solve and show all steps and calculation Equilibrium Constant Expressions Select the correct expression for the equilibrium constant for each of the following reaction
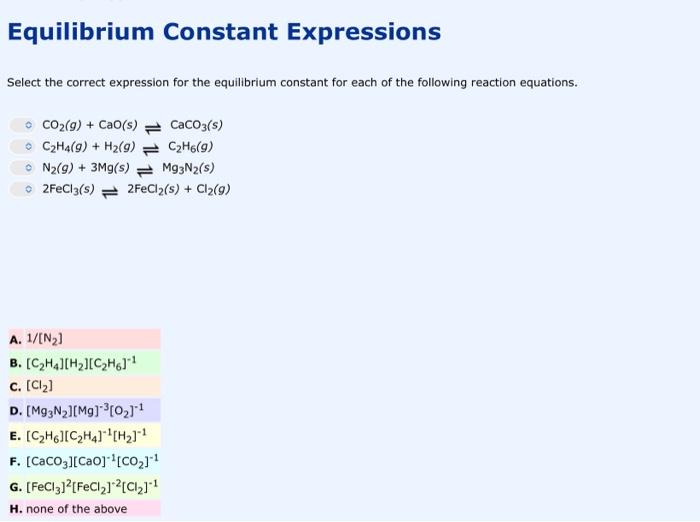
Equilibrium Constant Expressions Select the correct expression for the equilibrium constant for each of the following reaction equations. CO2(g)+CaO(s)CaCO3(s)C2H4(g)+H2(g)C2H6(g)N2(g)+3Mg(s)Mg3N2(s)2FeCl3(s)2FeCl2(s)+Cl2(g) A. 1/[N2] B. [C2H4][H2][C2H6]1 C. [Cl2] D. [Mg3N2][Mg]3[O2]1 E. [C2H6][C2H4]1[H2]1 F. [CaCO3][CaO1[CO2]1 G. [FeCl3]2[FeCl2]2[Cl2]1 H. none of the above
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


