Question: please solve asap i. D-Fructose cyclize to form hemiacetals. Write the chemical reaction and show which form is more stable and why? ii. Glucose can
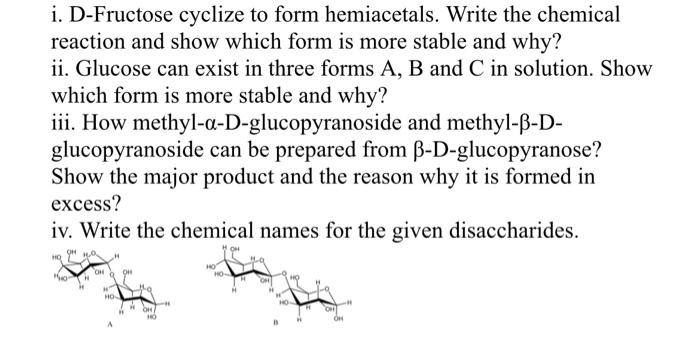
i. D-Fructose cyclize to form hemiacetals. Write the chemical reaction and show which form is more stable and why? ii. Glucose can exist in three forms A, B and C in solution. Show which form is more stable and why? iii. How methyl-a-D-glucopyranoside and methyl-B-D- glucopyranoside can be prepared from B-D-glucopyranose? Show the major product and the reason why it is formed in excess? iv. Write the chemical names for the given disaccharides. OH HO OM H 011 HO OH A i. D-Fructose cyclize to form hemiacetals. Write the chemical reaction and show which form is more stable and why? ii. Glucose can exist in three forms A, B and C in solution. Show which form is more stable and why? iii. How methyl-a-D-glucopyranoside and methyl-B-D- glucopyranoside can be prepared from B-D-glucopyranose? Show the major product and the reason why it is formed in excess? iv. Write the chemical names for the given disaccharides. OH HO OM H 011 HO OH A
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


