Question: Please, solve both by Polymath and by hand! Fi = 1000 kmol/h The gas mixture used for the synthesis of ammonia is obtained by mixing
Please, solve both by Polymath and by hand!
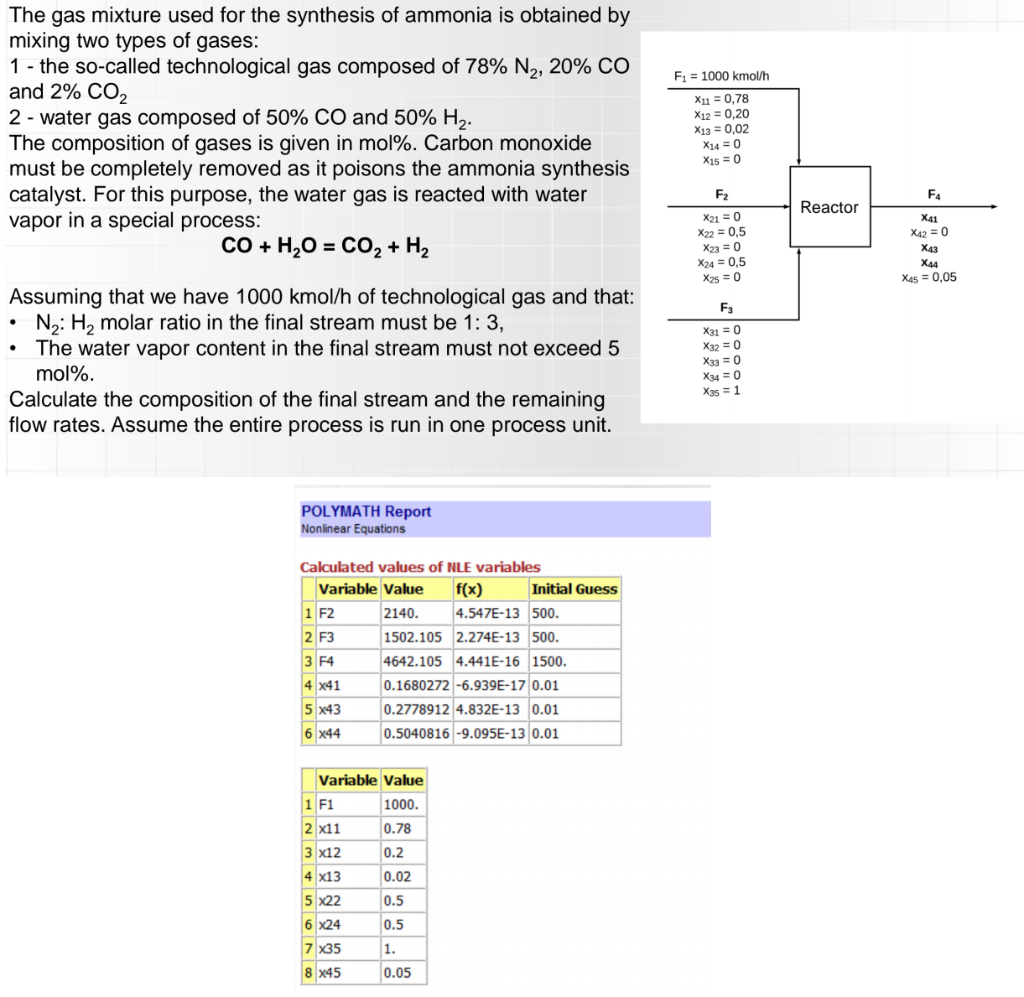
Fi = 1000 kmol/h The gas mixture used for the synthesis of ammonia is obtained by mixing two types of gases: 1 - the so-called technological gas composed of 78% N2, 20% CO and 2% CO2 2 - water gas composed of 50% CO and 50% H2. The composition of gases is given in mol%. Carbon monoxide must be completely removed as it poisons the ammonia synthesis catalyst. For this purpose, the water gas is reacted with water vapor in a special process: CO + H2O = CO2 + H2 X11 = 0,78 X12 = 0,20 X13 = 0,02 X14 = 0 X15 = 0 F2 F4 Reactor X21 = 0 X22 = 0,5 X23 = 0 X24 = 0,5 X2 = 0 X41 X42 = 0 X43 X44 X 5 = 0,05 F3 . . Assuming that we have 1000 kmol/h of technological gas and that: Nz: Hmolar ratio in the final stream must be 1: 3, The water vapor content in the final stream must not exceed 5 mol%. Calculate the composition of the final stream and the remaining flow rates. Assume the entire process is run in one process unit. X31 = 0 X32 = 0 X33 = 0 X34 = 0 X35 = 1 POLYMATH Report Nonlinear Equations Calculated values of NLE variables Variable Value f(x) Initial Guess 1 F2 2140. 4.547E-13 500. 2 F3 1502.105 2.274E-13 500. |3F4 4642.105 4.441E-16 1500. 4 X41 0.1680272 -6.939E-17 0.01 5 x43 0.2778912 4.832E-13 0.01 6 x44 0.5040816 -9.095E-13 0.01 Variable Value 1 F1 1000. 2 x11 0.78 3 x12 0.2 4 x13 0.02 5 X22 0.5 6 X24 0.5 7 X35 1. 8 X45 0.05
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


